Amity University-Noida B.Tech Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
JEE Main Chemistry sample paper with answer key - To prepare well for chemistry in the JEE Main 2026 exam, it is necessary first to understand what type of content to study, the exam format, and what kinds of questions will be asked. To assist you in your chemistry preparation, we have provided you with a sample paper for JEE Main 2026. This JEE Main 2026 Chemistry sample paper with answer key is designed based on the new NTA format, and will simulate the types of questions you may see in the test and help you assess how well you know the concepts and how quickly you can solve problems.
The National Testing Agency (NTA) will conduct the JEE Mains 2026 session 1 from April 2 to 9 and the result will be declared by April 20.
This Story also Contains

Along with the sample paper of JEE Main 2026 Chemistry, you will receive a complete answer key, which includes detailed solutions for each question, enabling you to check your performance and understand how to answer each question correctly. In the next sections, you will find the link to the entire sample paper and you can download the sample paper to practice. Now let us get started with the JEE Main 2026 Chemistry sample paper with the answer key.
The JEE Main 2026 Chemistry Sample Paper with Answer Key is created to help students practice exam-oriented questions and improve their understanding of both Physical, Organic, and Inorganic Chemistry. We are also giving the direct link so you can access, download, and solve the sample paper with ease.
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 25th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
For additional JEE Mains chemistry Sample Paper with Solutions 2026, students can use the free Careers360 resource. It provides mock tests, sample papers, chapter-wise and subject-wise tests, plus JEE Main Free Study Material.
1. Simulating actual Examination Conditions
Simulating actual Examination Conditions by solving JEE Main 2026 Chemistry sample paper with answer key using a timer similar to the one used in the actual JEE Main Examination helps to develop speed, accuracy, and examination temperament.
2. Identifying Your Weak Chapters
After attempting the Sample Papers, look at the topics that you performed poorly on, such as: Chemical Bonding, Thermodynamics, Organic Reaction Mechanisms, etc., and revise those specific chapters before proceeding to the next set of Sample Papers.
3. Understanding Why Your Answers Were Correct or Incorrect
Go through the JEE Mains chemistry Sample Paper with Solutions 2026 to fully understand the rationale behind each answer, whether it was correct or incorrect. Understanding why you answered questions incorrectly will help you avoid making the same mistakes again.
4. Creating Notes of Formulas & Reactions
While going through the JEE Main Chemistry sample paper with answer key 2026, make sure to add any important formulas, patterns in reactions, exceptions, or shortcut techniques to your notes.
5. Monitoring Your Improvement
While practicing with the JEE Main Chemistry sample paper with answer key 2026, continue to take at least one or two Sample Papers each week and compare your scores to determine your continued improvement and develop self-confidence.
Download ebook here:
We have given some questions from the Physics sample paper for reference. Here you can check the JEE Main 2026 Physics sample paper with answer key:
Identify the correct statement among the following:
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
98% Placement Record | Highest CTC 81.25 LPA | NAAC A++ Accredited | Ranked #62 in India by NIRF Ranking 2025 | JEE & JET Scores Accepted
Option 1: All naturally occurring amino acids except glycine contain one chiral centre. Option 2: All naturally occurring amino acids are optically active.
Option 3: Glutamic acid is the only amino acid that contains a –COOH group at the side chain.
Option 4: Amino acid cysteine easily undergoes dimerization due to the presence of free SH group.
Correct Answer: Amino acid, cysteine easily undergo dimerization due to the presence of free SH group.
Solution: * Isoleucine has 2 chiral centre
* Glycine is optically inactive
* Aspartic acid also contains COOH groups at the side chain.
* Cysteine easily dimmerise due to free SH gro
Consider the following molecules :
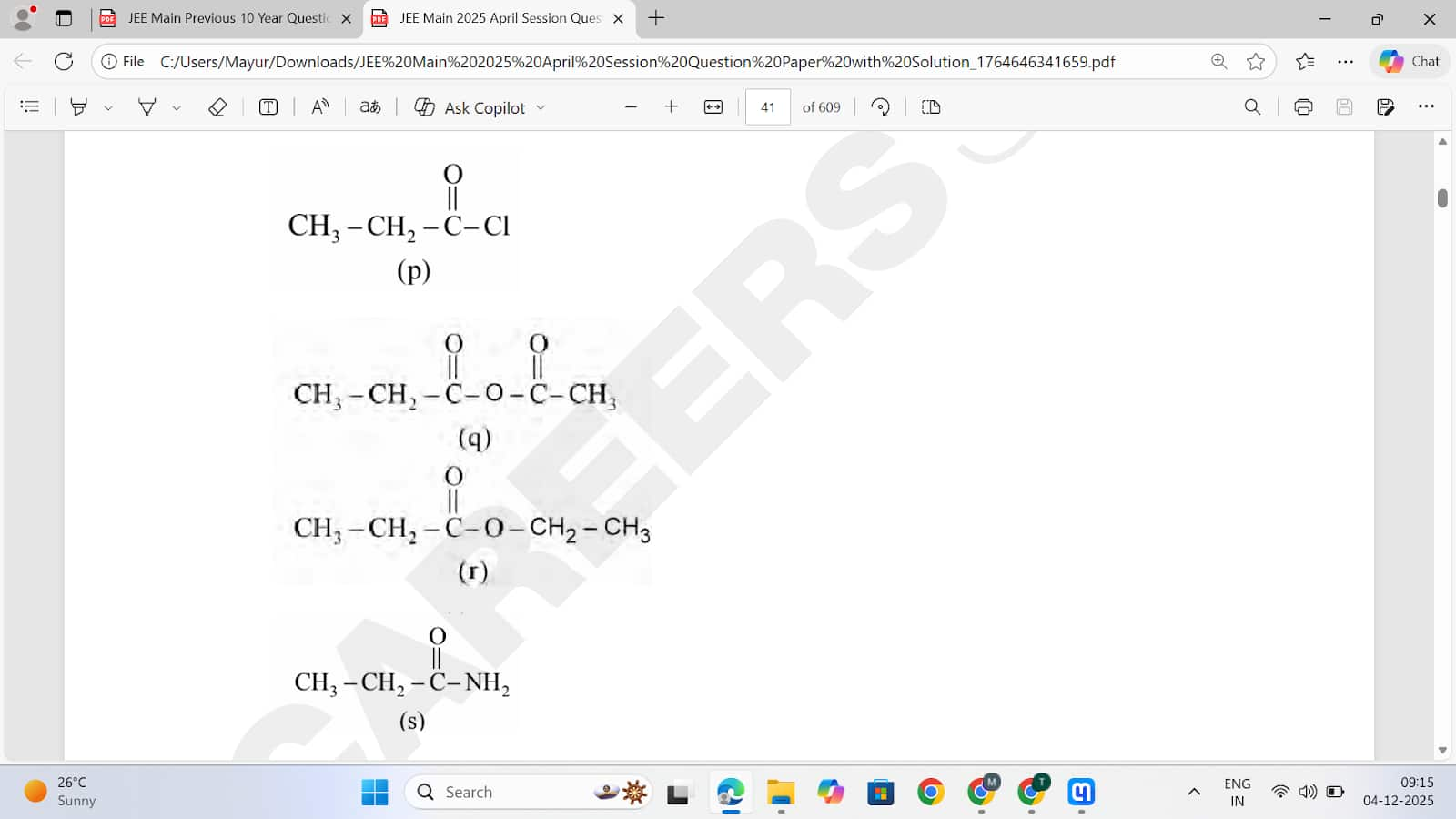
The correct order of rate of hydrolysis is
Option 1: r >q>p>s
Option 2: q >p>r>s 41
Option 3: p >r>q>s
Option 4: p >q>r>s Correct
Answer: p >q>r>s
Solution: Rate of hydrolysis ∝ Leaving group ability
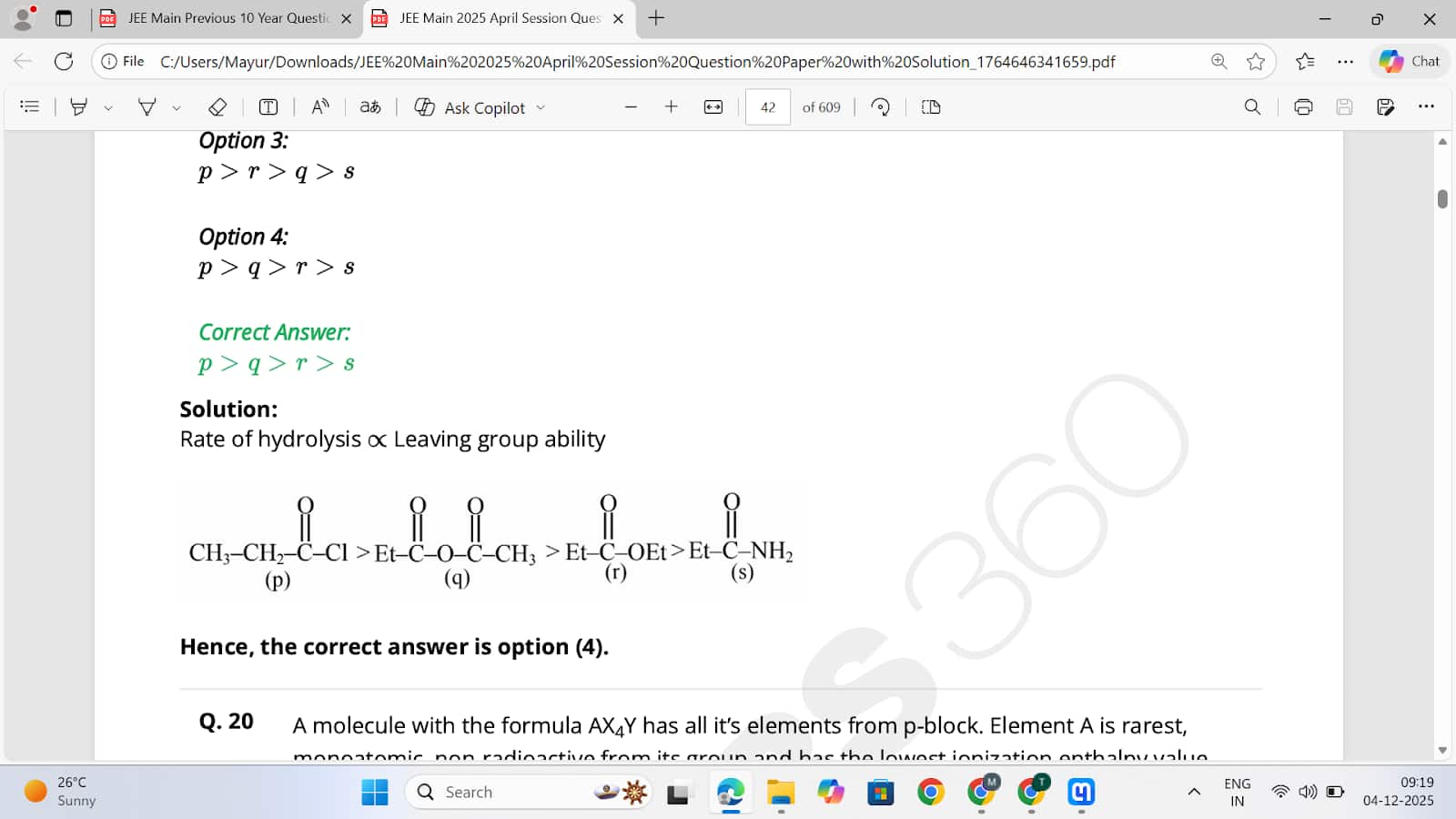
Hence, the correct answer is option (4).
A solution is made by mixing one mole of volatile liquid A with 3 moles of volatile liquid B. The vapour pressure of pure A is 200 mm Hg and that of the solution is 500 mm Hg. The vapour pressure of pure B and the least volatile component of the solution, respectively, are :
Option 1: 1400 mm Hg, A
Option 2: 1400 mm Hg, B
Option 3: 600 mm Hg, B
Option 4: 600 mm Hg, A
Correct Answer: 600 mm Hg, A
Solution:
$
\begin{aligned}
&\begin{aligned}
& P_S=P_A^{\circ} \cdot X_A+P_B^{\circ} \cdot X_B \
& 500=200 \times \frac{1}{4}+P_B^{\circ} \cdot \frac{3}{4} \
& P_B^{\circ}=600 \mathrm{mmHg}
\end{aligned}\
&\text { As } \mathrm{P}_{\mathrm{A}}^{\mathrm{o}}<\mathrm{P}_{\mathrm{B}}^0 \Rightarrow \mathrm{~A} \text { is least volatile. }
\end{aligned}
$
Hence, the correct answer is option (4).
The property/properties that show irregularity in first four elements of group-17 is/are :
(A) Covalent radius
(B) Electron affinity
(C) Ionic radius
(D) First ionization energy
Choose the correct answer from the options given below:
Option 1: B and D only
Option 2: A and C only
Option 3: B only
Option 4: A, B, C and D
Correct Answer: B only
Solution: The order of first four elements of group-17 are as follows
F< Cl< Br< I (Covalent radius)
Cl > F>Br>I (Electron affinity)
$
\mathrm{F}^{-}<\mathrm{Cl}^{-}<\mathrm{Br}^{-}<\mathrm{I}^{-}
$
F >Cl>Br>I ( Ist ionization energy)
Electron affinity order is irregular. Hence, the correct answer is option (3).
Download ebooks here:
Frequently Asked Questions (FAQs)
Yes, majorly the sample papers are made following the latest NTA exam pattern, which includes MCQs and numerical value-type questions.
Check accuracy, time spent per question, types of mistakes that can be conceptual or calculation, and topics where you repeatedly lose marks.
On Question asked by student community
There are many NITs that accepts JEE Main rank below 35,000 in different branches including Civil Engineering, Chemical Engineering, and many more. These NITs are -
Arnav Gautam & P.Mohith secured 300 out of 300 in JEE Mains session 1, as per the provisional answer key. The list of toppers to be released with JEE Main results
JEE Main 2026 session 1 result is not declared yet. JEE Main session 1 result will be declared on February 12.
A rank above 50,000 is considered good in JEE Mains. Some of the private colleges accepting JEE Mains are
The 69-77 marks in JEE Main examination are considered to be the average score and does not guarantee best branches in top tier NITs and IIITs. Some of the branches in which students with this much score can get admission are Electronics and Communication Engineering, Computer Science and Engineering, and
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
India's youngest NAAC A++ accredited University | NIRF rank band 151-200 | 2200 Recruiters | 45.98 Lakhs Highest Package
Last Date to Apply: 25th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
Top international universities | Know fees, location, courses offered.
NAAC A++ Grade | Recognized as Category-1 Deemed to be University by UGC | 41,000 + Alumni Imprints Globally