GNA University B.Tech Admissions 2026
100% Placement Assistance | Avail Merit Scholarships | Highest CTC 43 LPA
JEE Main 2026: The National Testing Agency has closed the JEE Main 2026 form correction window. Now, the JEE Main 2026 session 2 city intimation slip will be released in March 2026. The city intimation link will be activated on the official website, jeemain.nta.nic.in. To download the JEE Main city intimation slip, enter the JEE application number and password. After the intimation slip, the authority will issue the JEE Main 2026 session 2 admit card in March 2026. The JEE Main 2026 April session will be held from April 2 to 9, 2026.
Also Check- JEE Main 2026 Paper 2 Result
This Story also Contains

There are 12 JEE Main 2026 Session 1 toppers. Changes during the JEE Main 2026 form corrections were allowed from February 27 to 28,2026. The session 2 JEE Main 2026 registration was conducted from February 1 to 25, 2026. The memory-based JEE Main 2026 question papers are also available for previous shifts. Note that the Jan 23 shift 2 paper is being considered to be the toughest shift of the JEE Main 2026 session 1. Keep visiting the page for all major updates on JEE Mains city intimation slip, registration, admit card, syllanus, pattern and more.
JEE Mains exam is conducted for admission to top NITs, IIITs, and CFTIs in India. The JEE Mains exam also serves as a qualifying exam for the JEE Advanced exam, which is held for admission to the prestigious IITs. The JEE Main 2026 college predictor will help to predict the college that will be allotted based on the probable percentile. The National Testing Agency will conduct the Joint Entrance Examination Main 2026 in CBT Mode in two sessions.
Particulars | Details |
|---|---|
Examination Name | Joint Entrance Examination Main (JEE Main) |
Conducting Body | National Testing Agency (NTA) |
Purpose of Examination | Admission into NITs, IIITs, and CFTIs and a qualifying test for JEE Advanced |
Category | Undergraduate (UG) Exam |
Level of Exam | National Level |
Number of Sessions | 2 |
NTA JEE Main 2026 Exam Mode | Paper-1: Computer-based test Paper-2: |
Mode of Application | Online |
Official Web Portal | jeemain.nta.nic.in, jeemain.nta.ac.in and nta.ac.in |
| JEE Main 2026 city intimation slip date | Session 1- January 8, 2026 |
| JEE Mains admit card date 2026 | Session 1- January 25, 2026, for Jan 28 & 29 exam |
| JEE Main 2026 exam date | Session 1- Paper 1- January 21, 22, 23, 24 and 28 , 2026 |
NTA will release the JEE Main session 2 city slip 2026 online. Using the application number and password, the JEE Mains 2026 city intimation slip can be downloaded. Candidates get to know the exam centre city and the date of the test allotted to them. Note that the city slip of JEE Mains 2026 is an important document. However, candidates need not carry it to the exam centre. Only the JEE admit card 2026 and photo ID are the required documents on the exam day.
The following details will be mentioned on the JEE city intimation slip
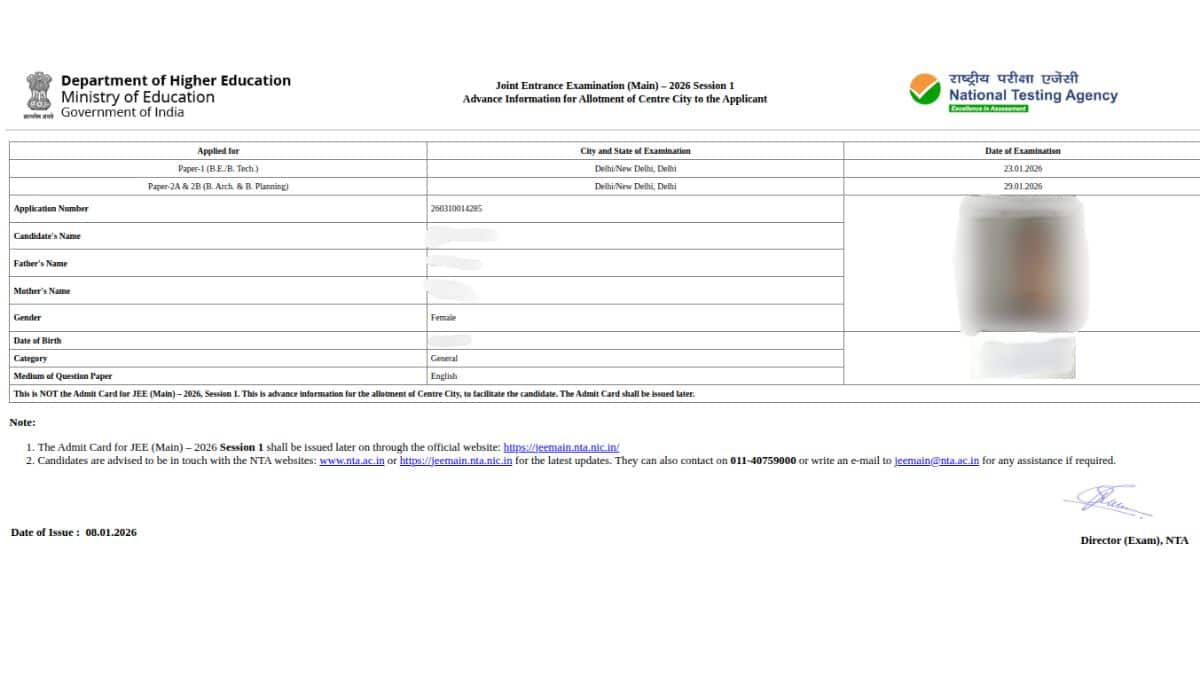
Below are the steps to download the NTA JEE Mains city intimation slip.
The National Testing Agency (NTA) conducted the JEE Main 2026 session 2 form correction window from February 27 to 28, 2026. The JEE Mains 2026 registration for session 2 was concluded from February 1 to 25, 2026. Candidates who meet the minimum eligibility criteria were able to register and complete the application process. The application process consists of registration, form filling, uploading documents, and payment of registration fee. The process includes the following steps:
Registration
Filling out the application form
Uploading required documents
Payment of the application fee
Registration: Before filling out the application form, candidates need to complete the registration process. During registration, candidates have to provide their details such as their name, guardian's name, gender, identification number, communication details, and more.
Filling form: After completing the JEE registration, candidates have to fill out the application form. In this process, candidates have to provide details such as contact, personal information, Aadhar, exam and centre, education qualification, and additional details.
Upload photograph: Candidates need to upload necessary documents such as photograph, signature, address proof, category certificate, and PwD certificate.
Fee Payment: This is the final step. Candidates have to pay the application fee through credit card, debit card, net banking, and UPI. After the successful transaction, the JEE Main application form will be submitted.
Category | BE/B.Tech or BArch or B.Planning | BE/B.Tech & BArch or BE/B.Tech & BPlanning or BE/B.Tech. B.Arch & B.Planning or B.Arch & B.Planning |
|---|---|---|
General | For Males- Rs 1000 For Females- Rs 800 | For Males- Rs 2000 For Females-Rs 1600 |
EWS/OBC-NCL | For Males- Rs 900 For Females- Rs 800 | For Males- Rs 2000 |
SC/ST/PWD/Transgender | For All- Rs 500 | For All- Rs 1000 |
Category | BE/B.Tech or BArch or B.Planning | BE/B.Tech & BArch or BE/B.Tech & BPlanning or BE/B.Tech. B.Arch & B.Planning or B.Arch & B.Planning |
|---|---|---|
General | For Males- Rs 5000 For Females- Rs 4000 | For Males- Rs 10000 For Females- Rs 8000 |
EWS/OBC-NCL | For Males - Rs. 4500 For Females- Rs. 4000 | For All- Rs 5000 |
SC/ST/PWD/Transgender | M / F- Rs 2500 Transgender - Rs. 3000 | For All- Rs 5000 |
NTA has released the JEE Main eligibility criteria 2026 along with the information brochure. The eligibility criteria are the conditions that candidates must fulfil to apply for the JEE entrance exam. Candidates who have passed the class 10+2 in 2024, and 2025, and appear for 2026 were able to register for the JEE Mains 2026. Moreover, candidates have to fulfil the other eligibility criteria for JEE Mains 2026. Below is the eligibility criteria for JEE Main 2026.
Candidates attempting the JEE Main exam must be aware of the JEE Main minimum age criteria. As per the notification, there is no minimum age limit to attempt the JEE Main exam.
To apply for the JEE Main exam, candidates require a minimum of 75% aggregate percentage in their class 12th. Candidates will not be considered for admission if they fail to have a minimum of 75 percentile. However, SC/ST candidates only require 65%.
The JEE Main State Code of Eligibility is used to determine the Home state quota eligibility of the candidate during the JoSAA counselling. The JEE Main State Code of Eligibility indicates the state in which the candidate passed the qualifying exam, but not their residence.
Candidates can find the complete list of qualifying exams, which applicants have to clear in class 12th to be eligible to apply for the JEE Main exam
Class 12th final exam (10+2 system) conducted by any recognised Central/State Board in India, such as CBSE, CISCE (ISC), NIOS, and all state boards.
Intermediate or two-year Pre-University examination conducted by a recognised Board/University.
Final examination of the two-year course of the Joint Services Wing of the National Defence Academy (NDA).
Senior Secondary School Examination conducted by the National Institute of Open Schooling (NIOS) with a minimum of five subjects.
Any Public School/Board/University Examination in India or abroad recognised as equivalent to the 10+2 system by the Association of Indian Universities (AIU).
Higher Secondary Certificate Vocational Examination.
Diploma recognized by AICTE or a State board of technical education of at least 3 years’ duration (Eligible for JEE Main but not for JEE Advanced.)
General Certificate Education (GCE) examination at the Advanced (A) level taken in the UK or Cambridge.
High School Certificate Examination of Cambridge University or International Baccalaureate Diploma of the International Baccalaureate Office, Geneva.
NTA will issue the JEE Main session 2 admit card 2026 to the registered candidates on the official website. JEE Mains admit card is an important document, without which, the candidates won't be allowed to enter the examination hall. The JEE Main 2026 admit card consisst of all the important information such as exam date and time, centre address, guidelines, and more. Follow the steps mentioned below for quickly download the NTA JEE Mains admit card.
NTA has introduced several changes in the JEE Mains 2026. The complete details have been provided in the JEE Mains information brochure. It includes updates about registration dates, application process, JEE document required, syllabus, exam dates, paper pattern and general instructions. Any new changes in the JEE Main 2026 exam have been included in the JEE Main NTA information brochure.
Keep checking the official website for the JEE Main 2026 latest updates. Knowing about the new changes in the JEE Main exam helps candidates plan their preparation more effectively and avoid last-minute surprises.
The National Testing Agency has announced the JEE Main exam date 2026 on the official website, jeemain.nta.nic.in 2026. Candidates can check the JEE exam date 2026 to know the schedule for important events such as registration, exam date, admit card, result, and more. However, the final JEE Main exam date January session and the April session to be updated along with the city slip. The JEE 2026 exam date is updated below.
| Event | Session 2 Date |
|---|---|
JEE Main 2026 registration date session 2 | February 1, 2026 |
JEE Main 2026 NTA registration last date | February 25, 2026 |
| JEE Main form correction 2026 release date | Session 2: February 27, 2026 |
| JEE Main form correction 2026 last date | Session 2: February 28, 2026 |
JEE Main admit card release date | March 2026 (3-4 days before the exam) |
JEE Main 2026 Session 2 Exam Date | April 2 to 09, 2026 |
Result Declaration Date | By 20 April, 2026 |
| Event | Session 1 Date |
|---|---|
Release of JEE Main 2026 official notification | October 31, 2025 |
JEE Mains registration date 2026 | October 31, 2025 |
JEE Mains 2026 registration last date session 1 | November 27, 2025 (till 9 PM) |
| JEE Main form correction 2026 release date | December 1, 2025 |
| JEE Main 2026 form correction last date | December 2, 2025 |
| City Intimation slip release | January 8, 2026 |
Release date for JEE Main exam admit card | January 25, 2026 ( for Jan 28 & 29 exam ) January 17, 2026, (For Jan 21 to 24 exams) |
JEE Main 2026 Session 1 Exam Date | January 21, 22, 23, 24, 28 and 29, 2026 January 23 exam has been postponed for the West Bengal exam centres |
| JEE Main 2026 answer key | Paper 1- February 4 to 5, 2026 Paper 2- February 19 to 20, 2026 |
JEE exam result release date | By |
The authority is expected to change the exam mode of the JEE Main exam from online to offline. The parliamentary panel of the JEE Main examination is discussing the mode of exam as students are facing technical problems in the online mode. The complete information on the JEE Main exam mode will be updated here whenever it is confirmed. In online mode, out-of-syllabus questions, incorrect answers, and technical problems have arisen previously. Due to this reason, the authority is meeting over whether to change the mode of the exam or not.
NTA has uploaded the JEE Main official notification at the official website, jeemain.nta.nic.in. After the release of the NTA JEE Mains 2026 notification, candidates can know the exam eligibility criteria, exam date, availability of the admit card, exam syllabus, paper pattern, and more. Aspirants are advised to visit the official website of JEE Main 2026 regularly for the latest information.
The syllabus of JEE Main 2026 is based on the NCERT syllabus for classes 11 and 12. JEE Main 2026 syllabus comprises of topics based on Physics, Chemistry and Mathematics. NTA released the final JEE Mains 2026 syllabus pdf at its official website, jeemain.nta.ac.in. Candidates must follow the JEE exam syllabus to prepare for the entrance exam.
| Chapters | Topics |
|---|---|
Units and Measurement | Units of Measurements, System of Units, S I Units, fundamental and derived units, least count, significant figures, Errors in measurements, Dimensions of Physics quantities, dimensional analysis, and its applications |
Kinematics | The frame of reference, motion in a straight line, Position- time graph, speed and velocity; Uniform and non-uniform motion, average speed and instantaneous velocity, uniformly accelerated motion, velocity-time, position-time graph, relations for uniformly accelerated motion. Relative Velocity, Motion in A Plane, Projectile Motion, Uniform Circular Motion. |
Force and inertia, Newton’s First Law of motion; Momentum, Newton’s Second Law of Motion, Impulses; Newton’s Third Law of motion. Law of conservation of linear momentum and its applications. Equilibrium of concurrent forces. Static and Kinetic friction, laws of friction, rolling friction. Dynamics of uniform circular motion: centripetal force and its applications: vehicle on a level circular road, vehicle on a banked road. | |
Work done by a constant force and a variable force; kinetic and potential energies, work-energy theorem, power. The potential energy of spring conservation of mechanical energy, conservative and nonconservative forces; motion in a vertical circle: Elastic and inelastic collisions in one and two dimensions. | |
Centre of the mass of a two-particle system, Centre of the mass of a rigid body; Basic concepts of rotational motion; moment of a force; torque, angular momentum, conservation of angular momentum and its applications; The moment of inertia, the radius of gyration, values of moments of inertia for simple geometrical objects, parallel and perpendicular axes theorems, and their applications. Equilibrium of rigid bodies, rigid body rotation and equations of rotational motion, comparison of linear and rotational motions. | |
The universal law of gravitation. Acceleration due to gravity and its variation with altitude and depth. Kepler’s law of planetary motion. Gravitational potential energy; gravitational potential. Escape velocity, Motion of a satellite, orbital velocity, time period, and energy of satellite. | |
Elastic behaviour, Stress-strain relationship, Hooke's Law. Young's modulus, bulk modulus, and modulus of rigidity. Pressure due to a fluid column; Pascal's law and its applications. Effect of gravity on fluid pressure. Viscosity. Stokes' law. terminal velocity, streamline, and turbulent flow.critical velocity. Bernoulli's principle and its applications. Surface energy and surface tension, angle of contact, excess of pressure across a curved surface, application of surface tension - drops, bubbles, and capillary rise. Heat, temperature, thermal expansion; specific heat capacity, calorimetry; change of state, latent heat. Heat transfer conduction, convection, and radiation. | |
Thermal equilibrium, zeroth law of thermodynamics, the concept of temperature. Heat, work, and internal energy. The first law of thermodynamics, isothermal and adiabatic processes. The second law of thermodynamics: reversible and irreversible processes. | |
Equation of state of a perfect gas, work done on compressing a gas, Kinetic theory of gases - assumptions, the concept of pressure. Kinetic interpretation of temperature: RMS speed of gas molecules: Degrees of freedom. Law of equipartition of energy and applications to specific heat capacities of gases; Mean free path. Avogadro's number. | |
Oscillations and periodic motion – time period, frequency, displacement as a function of time. Periodic functions. Simple harmonic motion (S.H.M.) and its equation; phase: oscillations of a spring -restoring force and force constant: energy in S.H.M. - Kinetic and potential energies; Simple pendulum - derivation of expression for its time period: Wave motion. Longitudinal and transverse waves, speed of the travelling wave. Displacement relation for a progressive wave. Principle of superposition of waves, reflection of waves. Standing waves in strings and organ pipes, fundamental mode, and harmonics. Beats. | |
Electrostatic | Electric Charges And Fields: Conservation of charge. Coulomb's law forces between two point charges, forces between multiple charges: superposition principle and continuous charge distribution. Electric field: Electric field due to a point charge, Electric field lines. Electric dipole, Electric field due to a dipole. Torque on a dipole in a uniform electric field. Electric flux. Gauss's law and its applications to find field due to infinitely long uniformly charged straight wire uniformly charged infinite plane sheet, and uniformly charged thin spherical shell. Electric potential and its calculation for a point charge, electric dipole and system of charges; potential difference, Equipotential surfaces, Electric Potential And Capacitance, energy of a system of two point charges and electric dipole in an electrostatic field. Conductors and insulators. Dielectrics and electric polarization, capacitors and capacitances, the combination of capacitors in series and parallel, and capacitance of a parallel plate capacitor with and without dielectric medium between the plates. Energy stored in a capacitor. |
Electric current. Drift velocity, mobility, and their relation with electric current. Ohm's law. Electrical resistance. V-l characteristics of Ohmic and non-ohmic conductors. Electrical energy and power. Electrical resistivity and conductivity. Series and parallel combinations of resistors; Temperature dependence of resistance. Internal resistance, potential difference, and emf of a cell, a combination of cells in series and parallel. Kirchhoff’s laws and their applications. Wheatstone bridge. Metre Bridge. | |
Magnetic Effects of Current & Magnetism | Biot - Savart law and its application to the current carrying circular loop. Ampere's law and its applications to infinitely long current carrying straight wire and solenoid. Moving Charges And Magnetism- Force on a moving charge in uniform magnetic and electric fields. Force on a current-carrying conductor in a uniform magnetic field. The force between two parallel currents carrying conductors-definition of ampere. Torque experienced by a current loop in a uniform magnetic field: Moving coil galvanometer, its sensitivity, and conversion to ammeter and voltmeter. Current loop as a magnetic dipole and its magnetic dipole moment. Bar magnet as an equivalent solenoid, magnetic field lines; Magnetic field due to a magnetic dipole (bar magnet) along its axis and perpendicular to its axis. Torque on a magnetic dipole in a uniform magnetic field. Para-, dia- and ferromagnetic substances with examples, the effect of temperature on magnetic properties |
Electromagnetic Induction and Alternating Current | Electromagnetic induction: Faraday's law. Induced emf and current: Lenz’s Law, Eddy currents. Self and mutual inductance. Alternating currents, peak and RMS value of alternating current/ voltage: reactance and impedance: LCR series circuit, resonance: power in AC circuits, wattless current. AC generator and transformer. |
Displacement current. Electromagnetic waves and their characteristics, Transverse nature of electromagnetic waves, Electromagnetic spectrum (radio waves, microwaves, infrared, visible, ultraviolet. X-rays. Gamma rays), Applications of e.m. waves | |
Reflection of light, spherical mirrors, mirror formula. Refraction of light at plane and spherical surfaces, thin lens formula, and lens maker formula. Total internal reflection and its applications. Magnification. Power of a Lens. Combination of thin lenses in contact. Refraction of light through a prism. Microscope and Astronomical Telescope (reflecting and refracting ) and their magnifying powers. Wave optics: wavefront and Huygens' principle. Laws of reflection and refraction using Huygens principle. Interference, Young's double-slit experiment, and expression for fringe width, coherent sources, and sustained interference of light. Diffraction due to a single slit, width of the central maximum. Polarisation, plane-polarised light: Brewster's law, uses of planepolarized light and Polaroid. | |
Dual nature of radiation. Photoelectric effect. Hertz and Lenard's observations; Einstein's photoelectric equation: particle nature of light. Matter waves-wave nature of particle, de Broglie relation. | |
Alpha-particle scattering experiment; Rutherford's model of atom; Bohr model, energy levels, hydrogen spectrum. Composition and size of nucleus, atomic masses, Mass-energy relation, mass defect; binding energy per nucleon and its variation with mass number, nuclear fission, and fusion | |
Semiconductors; semiconductor diode: I-V characteristics in forward and reverse bias; diode as a rectifier; I-V characteristics of LED. the photodiode, solar cell, and Zener diode; Zener diode as a voltage regulator. Logic gates (OR. AND. NOT. NAND and NOR). | |
Experimental Skills | Familiarity with the basic approach and observations of the experiments and activities:
|
| Chapters | Topics |
|---|---|
Matter and its nature, Dalton's Atomic Theory: Concept of atom, molecule, element, and compound, Laws of Chemical Combination, Atomic and molecular masses, Mole Concept, molar mass, percentage composition, empirical and molecular formulae: Chemical equations and stoichiometry | |
Nature of electromagnetic radiation, photoelectric effect; Spectrum of the hydrogen atom. Bohr model of a hydrogen atom - its postulates, derivation of the relations for the energy of the electron and radii of the different orbits, limitations of Bohr's model; Dual nature of matter, de Broglie's relationship. Heisenberg uncertainty principle. Elementary ideas of quantum mechanics, quantum mechanics, the quantum mechanical model of the atom, and its important features. Concept of atomic orbitals as one-electron wave functions: Variation of and 2 with r for 1s and 2s orbitals; various quantum numbers (principal, angular momentum, and magnetic quantum numbers) and their significance; shapes of s, p, and d - orbitals, electron spin, and spin quantum number: Rules for filling electrons in orbitals – Aufbau principle. Pauli's exclusion principle and Hund's rule, electronic configuration of elements, and extra stability of half-filled and completely filled orbitals. | |
Kossel-Lewis approach to chemical bond formation, the concept of ionic and covalent bonds. Ionic Bonding: Formation of ionic bonds, factors affecting the formation of ionic bonds; calculation of lattice enthalpy. Covalent Bonding: Concept of electronegativity. Fajan’s rule, dipole moment: Valence Shell Electron Pair Repulsion (VSEPR ) theory and shapes of simple molecules. Quantum mechanical approach to covalent bonding: Valence Bond Theory (VBT) - its important features, the concept of hybridization involving s, p, and d orbitals; Resonance. Molecular Orbital Theory - Its important features. LCAOs, types of molecular orbitals (bonding, antibonding), sigma and pi-bonds, molecular orbital electronic configurations of homonuclear diatomic molecules, the concept of bond order, bond length, and bond energy. Elementary idea of metallic bonding. Hydrogen bonding and its applications. | |
Fundamentals of thermodynamics: System and surroundings, extensive and intensive properties, state functions, Entropy, types of processes. The first law of thermodynamics - Concept of work, heat internal energy and enthalpy, heat capacity, molar heat capacity; Hess’s law of constant heat summation; Enthalpies of bond dissociation, combustion, formation, atomization, sublimation, phase transition, hydration, ionization, and solution. The second law of thermodynamics - Spontaneity of processes; S of the universe and G of the system as criteria for spontaneity. G (Standard Gibbs energy change) and equilibrium constant. | |
Electronic concepts of oxidation and reduction, redox reactions, oxidation number, rules for assigning oxidation number, and balancing of redox reactions. Electrolytic and metallic conduction, conductance in electrolytic solutions, molar conductivities and their variation with concentration: Kohlrausch’s law and its applications. Electrochemical cells - Electrolytic and Galvanic cells, different types of electrodes, electrode potentials including standard electrode potential, half-cell and cell reactions, emf of a Galvanic cell and its measurement: Nernst equation and its applications; Relationship between cell potential and Gibbs' energy change: Dry cell and lead accumulator; Fuel cells. | |
Rate of a chemical reaction, factors affecting the rate of reactions: concentration, temperature, pressure, and catalyst; elementary and complex reactions, order and molecularity of reactions, rate law, rate constant and its units, differential and integral forms of zero and first-order reactions, their characteristics and half-lives, the effect of temperature on the rate of reactions, Arrhenius theory, activation energy and its calculation, collision theory of bimolecular gaseous reactions (no derivation). | |
Different methods for expressing the concentration of solution - molality, molarity, mole fraction, percentage (by volume and mass both), the vapour pressure of solutions and Raoult's Law - Ideal and non-ideal solutions, vapour pressure - composition, plots for ideal and nonideal solutions; Colligative properties of dilute solutions - a relative lowering of vapour pressure, depression of freezing point, the elevation of boiling point and osmotic pressure; Determination of molecular mass using colligative properties; Abnormal value of molar mass, Van’t Hoff factor and its significance. | |
Equilibria involving physical processes: Solid-liquid, liquid-gas - gas and solid-gas equilibria, Henry's law. General characteristics of equilibrium involving physical processes. Equilibrium involving chemical processes: Law of chemical equilibrium, equilibrium constants (Kp and Kc) and their significance, factors affecting equilibrium concentration, pressure, temperature, the effect of catalyst; Le Chatelier’s principle. Ionic equilibrium: Weak and strong electrolytes, ionization of electrolytes, various concepts of acids and bases (Arrhenius. Bronsted - Lowry and Lewis) and their ionization, acid-base equilibria (including multistage ionization) and ionization constants, ionization of water. pH scale, common ion effect, hydrolysis of salts and pH of their solutions, the solubility of sparingly soluble salts and solubility products, and buffer solutions. |
| Chapters | Topics |
|---|---|
Modem periodic law and present form of the periodic table, s, p. d and f block elements, periodic trends in properties of elements atomic and ionic radii, ionization enthalpy, electron gain enthalpy, valence, oxidation states, and chemical reactivity. | |
Group -13 to Group 18 Elements General Introduction: Electronic configuration and general trends in physical and chemical properties of elements across the periods and down the groups; unique behaviour of the first element in each group. | |
Transition Elements General introduction, electronic configuration, occurrence and characteristics, general trends in properties of the first-row transition elements - physical properties, ionization enthalpy, oxidation states, atomic radii, colour, catalytic behaviour, magnetic properties, complex formation, interstitial compounds, alloy formation; Preparation, properties, and uses of K2Cr2O7, and KMnO4. Inner Transition Elements Lanthanoids - Electronic configuration, oxidation states, and lanthanoid contraction. Actinoids - Electronic configuration and oxidation states. | |
Introduction to coordination compounds. Werner's theory; ligands, coordination number, denticity. chelation; IUPAC nomenclature of mononuclear co-ordination compounds, isomerism; Bonding-Valence bond approach and basic ideas of Crystal field theory, colour and magnetic properties; Importance of co-ordination compounds (in qualitative analysis, extraction of metals, and in biological systems). |
| Chapters | Topics |
|---|---|
Purification And Characterisation Of Organic Compounds | Purification - Crystallization, sublimation, distillation, differential extraction, and chromatography - principles and their applications. Qualitative analysis - Detection of nitrogen, sulphur, phosphorus, and halogens. Quantitative analysis (basic principles only) - Estimation of carbon, hydrogen, nitrogen, halogens, sulphur, and phosphorus. Calculations of empirical formulae and molecular formulae: Numerical problems in organic quantitative analysis. |
Some Basic Principles Of Organic Chemistry | Tetravalency of carbon: Shapes of simple molecules - hybridization (s and p): Classification of organic compounds based on functional groups: and those containing halogens, oxygen, nitrogen, and sulphur; Homologous series: Isomerism - structural and stereoisomers. Nomenclature (Trivial and IUPAC) Covalent bond fission - Homolytic and heterolytic: free radicals, carbocations, and carbanions; stability of carbocations and free radicals, electrophiles, and nucleophiles. Electronic displacement in a covalent bond - Inductive effect, electromeric effect, resonance, and hyperconjugation. Common types of organic reactions - Substitution, addition, elimination, and rearrangement. |
Classification, isomerism, IUPAC nomenclature, general methods of preparation, properties, and reactions. Alkanes - Conformations: Sawhorse and Newman projections (of ethane): Mechanism of halogenation of alkanes. Alkenes - Geometrical isomerism: Mechanism of electrophilic addition: addition of hydrogen, halogens, water, hydrogen halides (Markownikoffs and peroxide effect): Ozonolysis and polymerization. Alkynes - Acidic character: Addition of hydrogen, halogens, water, and hydrogen halides: Polymerization. Aromatic hydrocarbons - Nomenclature, benzene - structure and aromaticity: Mechanism of electrophilic substitution: halogenation, nitration. Friedel-Craft's alkylation and acylation, directive influence of the functional group in monosubstituted benzene. | |
Organic Compounds Containing Halogens | General methods of preparation, properties, and reactions; Nature of C-X bond; Mechanisms of substitution reactions. Uses; Environmental effects of chloroform, iodoform freons, and DDT |
Organic Compounds Containing Oxygen | Alcohols: Identification of primary, secondary, and tertiary alcohols: mechanism of dehydration. Phenols: Acidic nature, electrophilic substitution reactions: halogenation. nitration and sulphonation. Reimer - Tiemann reaction. Ethers: Structure. Aldehyde and Ketones: Nature of carbonyl group; Nucleophilic addition to >C=O group, relative reactivities of aldehydes and ketones; Important reactions such as - Nucleophilic addition reactions (addition of HCN. NH3, and its derivatives), Grignard reagent; oxidation: reduction (Wolf Kishner and Clemmensen); the acidity of-hydrogen. aldol condensation, Cannizzaro reaction. Haloform reaction, Chemical tests to distinguish between aldehydes and Ketones. Carboxylic Acids Acidic strength and factors affecting it, |
Organic Compounds Containing Nitrogen | General methods of preparation. Properties, reactions, and uses. Amines: Nomenclature, classification structure, basic character, and identification of primary, secondary, and tertiary amines and their basic character. Diazonium Salts: Importance in synthetic organic chemistry. |
Biomolecules | General introduction and importance of biomolecules. Carbohydrates - Classification; aldoses and ketoses: monosaccharides (glucose and fructose) and constituent monosaccharides of oligosaccharides (sucrose, lactose, and maltose). Proteins - Elementary Idea of α-amino acids, peptide bond, polypeptides. Proteins: primary, secondary, tertiary, and quaternary structure (qualitative idea only), denaturation of proteins, enzymes. Vitamins– Classification and functions. Nucleic Acids – Chemical constitution of DNA and RNA. Biological functions of nucleic acids. Hormones (General introduction) |
Principles Related To Practical Chemistry | Detection of extra elements (Nitrogen, Sulphur, halogens) in organic compounds; Detection of the following functional groups; hydroxyl (alcoholic and phenolic), carbonyl (aldehyde and ketones) carboxyl, and amino groups in organic compounds. • The chemistry involved in the preparation of the following: Inorganic compounds; Mohr’s salt, potash alum.Organic compounds: Acetanilide, p-nitro acetanilide, aniline yellow, iodoform. • The chemistry involved in the titrimetric exercises – Acids, bases, and the use of indicators, oxalic-acid vs KMnO4, Mohr’s salt vs KMnO4 • Chemical principles involved in the qualitative salt analysis: Cations – Pb2+, Cu2+, Al3+, Fe3+, Zn2+, Ni2+, Ca2+, Ba2+, Mg2, NH+4 Anions- CO2−3, S 2- ,SO2−4, NO 3- , NO2- , Cl- , Br- , I- ( Insoluble salts excluded). Chemical principles involved in the following experiments: 1. Enthalpy of solution of CuSO4 2. Enthalpy of neutralization of strong acid and strong base. 3. Preparation of lyophilic and lyophobic sols. 4. Kinetic study of the reaction of iodide ions with hydrogen peroxide at room temperature. |
| Chapters | Topics |
|---|---|
Complex numbers as ordered pairs of reals, Representation of complex numbers in the form a + ib and their representation in a plane, Argand diagram, algebra of complex number, modulus, and argument (or amplitude) of a complex number, Quadratic equations in real and complex number system and their solutions Relations between roots and co-efficient, nature of roots, the formation of quadratic equations with given roots. | |
Matrices and Determinants | Matrices, algebra of matrices, type of matrices, determinants, and matrices of order two and three, evaluation of determinants, area of triangles using determinants, Adjoint, and evaluation of inverse of a square matrix using determinants and, Test of consistency and solution of simultaneous linear equations in two or three variables using matrices. |
Sets and their representation: Union, intersection, and complement of sets and their algebraic properties; Power set; Relation, Type of relations, equivalence relations, functions; one-one, into and onto functions, the composition of functions | |
The fundamental principle of counting, permutation as an arrangement and combination as section, Meaning of P (n,r) and C (n,r), simple applications. | |
Binomial theorem for a positive integral index, general term and middle term, and simple applications. | |
Arithmetic and Geometric progressions, insertion of arithmetic, geometric means between two given numbers, Relation between A.M and G.M. | |
Real–valued functions, algebra of functions, polynomials, rational, trigonometric, logarithmic, and exponential functions, inverse function. Graphs of simple functions. Limits, continuity, and differentiability. Differentiation of the sum, difference, product, and quotient of two functions. Differentiation of trigonometric, inverse trigonometric, logarithmic, exponential, composite, and implicit functions; derivatives of order up to two, Applications of derivatives: Rate of change of quantities, monotonic-Increasing and decreasing functions, Maxima and minima of functions of one variable, | |
Integral as an anti-derivative, Fundamental integral involving algebraic, trigonometric, exponential, and logarithmic functions. Integrations by substitution, by parts, and by partial functions. Integration using trigonometric identities. The fundamental theorem of calculus, properties of definite integrals. Evaluation of definite integrals, determining areas of the regions bounded by simple curves in standard form | |
Ordinary differential equations, their order, and degree, the solution of differential equation by the method of separation of variables, solution of a homogeneous and linear differential equation of the type | |
Coordinates of a point in space, the distance between two points, section formula, directions ratios, and direction cosines, and the angle between two intersecting lines. Skew lines, the shortest distance between them, and its equation. Equations of a line | |
Statistics And Probability | Measures of discretion; calculation of mean, median, mode of grouped and ungrouped data calculation of standard deviation, variance, and mean deviation for grouped and ungrouped data. Probability: Probability of an event, addition and multiplication theorems of probability, Baye's theorem, probability distribution of a random variate |
Vectors and scalars, the addition of vectors, components of a vector in two dimensions and three-dimensional space, scalar and vector products. | |
CO-ORDINATE GEOMETRY | Cartesian system of rectangular coordinates in a plane, distance formula, sections formula, locus, and its equation, the slope of a line, parallel and perpendicular lines, intercepts of a line on the co-ordinate axis. Straight line- Various forms of equations of a line, intersection of lines, angles between two lines, conditions for concurrence of three lines, the distance of a point form a line, co-ordinate of the centroid, orthocentre, and circumcentre of a triangle, Circle, conic sectio- A standard form of equations of a circle, the general form of the equation of a circle, its radius and central, equation of a circle when the endpoints of a diameter are given, points of intersection of a line and a circle with the centre at the origin and sections of conics, equations of conic sections (parabola, ellipse, and hyperbola) in standard forms, |
| Mathematics | Aptitude Test | Drawing Test | Planning (B. Plan) |
|---|---|---|---|
|
|
|
The JEE Main exam pattern helps candidates understand the structure of the question paper. It includes details such as the mode of exam, types of questions, marking scheme, total number of questions, and the language of the paper. JEE Main question paper is divided into two sections:
Section A: Contains 20 Multiple Choice Questions (MCQs)
Section B: Contains 10 Numerical Value Questions
Candidates must note that negative marking applies for incorrect answers.
| Particulars | Details |
|---|---|
Mode of exam | Online (Computer-Based Test) |
Subjects | Physics, Chemistry and Mathematics |
Type of questions | Multiple Choice Questions (MCQ) Numerical Value Answer (NAV) type questions |
Total number of questions | 75 Questions |
Exam Duration | 3 hours |
Subject-wise number of questions |
|
JEE Marking scheme |
|
JEE Mains Total Marks | 300 |
Language of paper | Assamese, Bengali, Marathi, Odia, Kannada, Malayalam, Punjabi, Tamil, Hindi, English, Telugu, and Urdu and Gujarati |
Particulars | Details |
|---|---|
Mode of exam | Online (Computer-based test) except drawing section |
Subjects covered | Mathematics, General Aptitude, Drawing |
Type of questions |
|
Total number of questions | 75+2 (drawing) |
Total Marks | 300 |
Subejct-wise number of questions |
|
Test Duration | 3 hours |
Marking scheme |
|
Language of paper | Assamese, Bengali, Marathi, Odia, Kannada, Malayalam, Punjabi, Tamil, Hindi, English, Telugu, and Urdu and Gujarati |
| Particulars | Details |
|---|---|
Mode of exam | Online (Computer-based test) |
Subjects covered | Mathematics, General Aptitude and Planning |
Total number of questions | 105 |
Total Marks | 300 |
Subject-wise number of questions |
|
Total duration of exam | 3 hours |
Type of questions | Multiple choice questions (MCQ) and Numerical Value Questions (NAV) in Mathematics Multiple choice questions (MCQ) in General Aptitude and Planning |
Marking scheme |
|
Language of paper | Assamese, Bengali, Marathi, Odia, Kannada, Malayalam, Punjabi, Tamil, Hindi, English, Telugu, and Urdu and Gujarati |
Solving previous year JEE Main question papers helps students understand the exam pattern, question difficulty, and frequently asked topics. These JEE PYQs are the best resources for practice and self-assessment. Download JEE Main previous year papers with solutions to improve your speed, accuracy, and confidence before the actual exam. NTA will release the official JEE Main question paper online.
| Session | Links |
|---|---|
| JEE Main 2025 January Session Official Question Paper with Detailed Solution | Download PYQs & Solution |
| JEE Main 2025 April Session Question Paper with Solution | Download Papers with Solution PDf |
| JEE Main 2024 January session | Download Question paper pdf with solutions |
| JEE Main 2024 April session | |
| JEE Main 2023 April Paper with Solutions | Download PYQs pdf |
| JEE Main 2023 January Paper with Solutions | Download PYQs pdf |
Aspirants must strategise their preparation effectively to score good marks. Below are some helpful preparation tips for JEE Main 2026.
The authority will activate JEE Main 2026 mock test for session 1 on the official website. The JEE Main exam mock tests will be prepared as per the JEE Mans 2026 syllabus and paper pattern. Candidates can practice JEE mock tests for free to familiarize themselves with the exam pattern, difficulty level, and syllabus. Practising the JEE Mains syllabus 2026 is crucial for achieving good marks in the exam.
Candidates can follow the detailed subject-wise study plan below to effectively prepare for JEE Main 2026.
NTA has released the session 1 JEE Main 2026 Paper 2 result on the official website. The authority declares the result for each session separately. A candidate's JEE Main scorecard will comprise the percentile score obtained in each subject. For candidates appearing in both sessions, the best score of the two will be considered for the final result and All India Rank. Candidates can follow the steps mentioned below for JEE Main result download.
Candidates can check the Marks vs Rank for JEE Main in the table below.
| Score out of 300 | Rank |
|---|---|
286- 292 | 19-12 |
280-284 | 42-23 |
268- 279 | 106-64 |
250- 267 | 524-108 |
231-249 | 1385-546 |
215-230 | 2798-1421 |
200-214 | 4667-2863 |
189-199 | 6664- 4830 |
175-188 | 10746-7152 |
160-174 | 16163-11018 |
149-159 | 21145-16495 |
132-148 | 32826-22238 |
120-131 | 43174-33636 |
110-119 | 54293-44115 |
102-109 | 65758-55269 |
95-101 | 76260-66999 |
89-94 | 87219-78111 |
79-88 | 109329-90144 |
62-87 | 169542-92303 |
41-61 | 326517-173239 |
1-40 | 1025009-334080 |
Below is the last 3 year's cut off marks for JEE Mains exam. Candidates must try to surpass the JEE cut off marks in order to get qualified for JEE Advanced.
| Category | 2025 | 2024 | 2023 |
|---|---|---|---|
General | 93.1023262 | 93.2362181 | 90.7788642 |
Gen-PwD | 0.0079349 | 0.00187 | 0.0013527 |
EWS | 80.3830119 | 81.3266412 | 75.6229025 |
OBC-NCL | 79.4313582 | 79.6757881 | 73.6114227 |
SC | 61.1526933 | 46.697584 | 51.9776027 |
ST | 47.9026465 | 60.0923182 | 37.2348772 |
NTA is the National Testing Agency. It is responsible for conducting several national-level entrance exams. One of the major entrance exams in JEE Main. NTA conducts JEE Mains on behalf of the Department of Higher Education, Ministry of Education. The Joint Entrance Examination Main is an All India level engineering entrance test for admission to NITs, IIITsand CFTIs. Moreover, it is a qualifying exam for JEE Advanced.
JEE Main is a national-level entrance exam for admission into top engineering colleges like NITs, IIITs, and other centrally funded institutions. Conducted by the NTA, it also serves as a qualifying test for JEE Advanced, required for IIT admissions. The exam tests candidates in Physics, Chemistry, and Mathematics and is held in multiple sessions each year. JEE Main exam is held for two papers - Paper 1 (BE/B.Tech), and Paper 2 (B.Arch/BPlan). Moreover, JEE Main is the gateway for JEE Advanced. The top 2, 50,000 JEE Mains qualifying candidates can apply for the JEE Advanced.
To get admission in one of the engineering colleges in India, candidates have to appear for JEE Mains counselling. Which is, also referred as JOSAA counselling. The complete schedule for JEE Main counselling will be issued after the declaration of JEE Advanced results, in May 2026.
A total of 31 National Institutes of Technology (NITs), 23 Indian Institutes of Information Technology (IIITs), and 40 other Government Funded Technical Institutes (GFTIs) offer admission based on the JEE Main exam.
| Name of the Institute | Total Number of Seats |
|---|---|
1112 | |
888 | |
1203 | |
1104 | |
1084 | |
480 | |
160 | |
1243 | |
360 | |
909 | |
188 | |
944 | |
751 | |
1147 | |
226 | |
165 | |
190 | |
203 | |
949 | |
275 | |
1159 | |
1066 | |
160 | |
903 | |
899 | |
956 | |
1038 | |
180 | |
989 | |
1325 | |
933 | |
| Indian Institutes of Engineering Science and Technology, Shibpur | 764 |
The official JEE Mains 2026 final answer key has been uploaded by NTA on February 16, 2026. Candidates can find below the unofficial answer key links of JEE Main Jan 21, 22, 23, 24 and 28 shifts.
JEE Main 2026 Answer Key January Session -
The official JEE Main 2026 paper will be released online by NTA. Careers360 has uploaded the memory-based JEE Main 2026 question papers for all shifts held till now. Students need to cross-check their given answers with the JEE Mains today's paper and know about their marks. Candidates can find below the question papers links of JEE Main Jan 21, 22, 23, 24 and 28 shifts.
JEE Main 2026 Question Paper with Solutions PDF-
JEE Main paper analysis is based on the JEE Main candidates' reactions 2026 and expert opinions. The JEE Mains 2026 difficulty level varies as per students' capabilities. The JEE Main 2026 BTech exam analysis shows that Chemistry was challenging. Physics was diffculty level that was also a bit on the higher side. Maths mostly had lengthy and short questions in equal proportion.
NTA has released the JEE Main 2026 response sheet, which shows the candidate’s marked responses during the exam. This is released alongside the provisional answer key to help students calculate the tentative scores.
Frequently Asked Questions (FAQs)
Yes, NTA JEE Main is being held in 2026. The JEE Main session 1 exam was conducted from Janury 21 to 29, and the session 2 exam is scheduled from April 2 to 9, 2026.
NTA has released the syllabus of JEE Main 2026 at the official website, jeemain.nta.ac.in. The syllabus of JEE Main 2026 is based on classes 11 & 12 Physics, Chemistry and Mathematics.
Yes, candidates can start their preparation for JEE 2026. Early preparation will be beneficial in cracking the JEE Mains exam.
According to last year's statistics, Calculus (25%), Mechanics, SHM, Electrostatics, Organic reaction mechanisms etc. are the high weightage topics.
NCERT are the best books to prepare with, however follow some other books like RD Sharma, HC Verma for better practice.
The JEE Mains 2026 registration for session 2 began on February 1, 2026.
To registration for the JEE Main 2026 exam, candidates can check the official website, jeemain.nta.nic.in.
The dates of examinations are as follows: Session 1: on 21, 22, 23, 24 and 28 January 2026 (paper 1), January 29, 2026 (paper 2) and Session 2: Between 02 April and 09 April 2026. JEE Main exam on January 23, 2026, will be rescheduled for the West Bengal candidates.
As per news reports, the number of candidates registered for JEE Main 2026 is around 14.15 lakhs. The authority will officially release the number of candidates who applied for JEE Mains 2026 online.
On Question asked by student community
Usha Mittal of Technology has no AI quota officially. please contact the college for any mangement quota seats.
Hello student,
Kindly go through the article to understand what a safe percentile is in JEE Main 2026 to qualify for JEE Advanced.
Link - JEE Main 2026 Advanced Qualifying Percentile
Hope this will be helpful!
To get admission, your JEE Main rank should be between 2 to 5 lakh.
For mode details visit- https://www.careers360.com/university/odisha-university-of-technology-and-research-bhubaneswar/cut-off
Hi !
I am sorry to hear that. I wish you the best this year.
Yes, you can apply for JEE Mains while you are in 12th class. While filling the application form, make sure you state clearly that you are 'Appearing' in 12th boards. You need to also make
decent chances actually as home state quota seats are 50%. allotments will depend on the JEE rank and not percentile though. in 2025, for female supernumerary it closed at 9286 rank while for open gen it closed at 5573.
So, would advise to use this tool to check the probable
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
Last Date to Apply: 15th March | NAAC A++ Accredited | Accorded institution of Eminence by Govt. of India | NIRF Rank #3
70th University Ranked by NIRF | 80th Engineering Rank by NIRF | Accredited by NBA and NAAC A+
Last Date to Apply: 26th March | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
Highest CTC 44.14 LPA | UGC Approved | 1600+ Recruiters | 100% Placement