GNA University B.Tech Admissions 2026
100% Placement Assistance | Avail Merit Scholarships | Highest CTC 43 LPA
JEE Main Chemistry Formulas 2026 - The Joint Entrance Exam is one of the most competitive exam of engineering entrance exams. It is divided into three subsections (Physics, Chemistry, Maths). Chemistry is an important subject for JEE Main preparation. Hence, having a list of important formulas for JEE Main Chemistry 2025 is necessary. Every aspirant should have the short notes for JEE Mains that comprise the important formulas. These JEE important formulas for Chemistry help in solving the typical problems easily. The registration process for JEE Main 2026 is now open, and students can aply from 31 October 2025 to 27 November 2025. Session 1 of the exam will be conducted from 21 to 29 January 2026.
This Story also Contains

Download the JEE Main Chemistry Formulas E-Book below:
JEE Mains Chemistry all Formulas PDF Download
There will be a total of 25 questions from Chemistry in JEE Main 2026, all of which have to be attempted. It is further segregated into Physical, Organic, and Inorganic sections. The maximum number of topics has been reduced from this section (chemistry) only. This makes Chemistry easier than before. However, the high-weightage topics for JEE Main remain the same.
Below are important formulas for JEE Main 2026 Chemistry that are helpful for both sessions. Candidates should solve as many Chemistry questions as they can and implement these formulas to remember them quickly. To crack the JEE Main exam with good marks, paste these formulas near the study table and memorise them.
Candidates while studying chemistry, they need to revise and practice the chemical equations and symbols. To some, chemistry is a tough subject, but when candidates practice chemical equations, revise the properties, formulas and symbols, they will have command over the subject. Candidates can check the JEE Main Chemistry formulas below
Some Basic Concepts in chemistry
Atomic Structure
Line Spectrum of Hydrogen-like atoms
Bohr radius of nth orbit:
Velocity of electron in nth orbit:
where Z is atomic number
Total energy of electron in nth orbit:
where Z is atomic number
Hisenberg Uncertainity Principle:
Chemical Thermodynamics
Work done in a reversible isothermal process
Work done in an irreversible isothermal process
Work
That is, Work
Enthalpy:
First Law of Thermodynamics:
Equilibrium
ELECTROCHEMISTRY
W or M = mass deposited
The emf can be calculated as
Solutions
Chemical kinetics
Unit of Rate Constant-
For the first-order reaction,
For any general nth order reaction it is evident that,
It is to be noted that the above formula is applicable for any general nth-order reaction except
Arrhenius Equation:
Coordination Compounds
Where:
L = Number of ligands (or donor atoms)
Crystal Field Stabilization Energy (CFSE):
Octahedral:
Tetrahedral:
where
d- & f-Block Elements
Magnetic Moment:
Chemical Bonding and Molecular Structure
Formal Charge:
(
Bond Order (Molecular Orbital Theory):
Dipole Moment:
(
Some Basic Principles of Organic Chemistry
Application of Inductive Effect
The decreasing -I effect or increasing +I effect order is as follows:
Degree of Unsaturation (DU or IHD):
(
Hydrocarbons
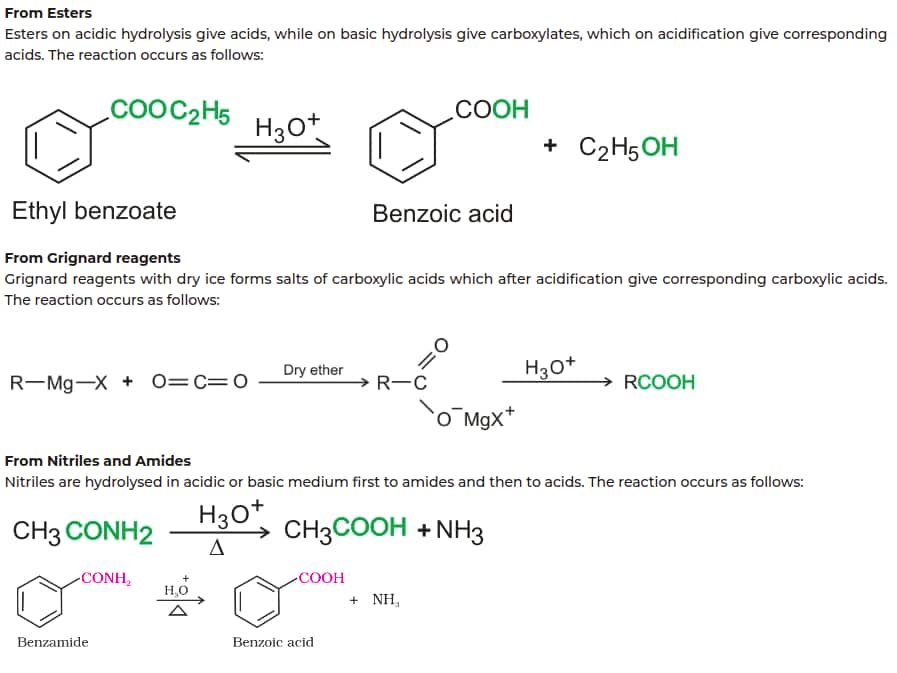
Carboxylic Acids and Derivatives
Method of Preparation of Carboxylic Acid
Given below are some tips to help you prepare for JEE Main and score good marks in the exam:
1. First, students need to understand the Syllabus and Exam Pattern so that they can refer to the JEE Main syllabus from the official website.
2. Try to identify the important and high-weightage topics and prepare according to that.
3. Create an effective study plan according to your preparation level. Divide your preparation into monthly, weekly, and daily targets and allocate more time to difficult subjects or topics.
4. Students must focus on conceptual clarity; they must understand the logic and derivations behind every formula.
5. Try to solve questions regularly. Solve previous years' JEE Main question papers and attempt mock tests and sample papers regularly.
Students find it difficult to learn formulas for JEE Main, but with the right approach, they can remember them. Given below are some points to remember:
1. Students must try to understand why a formula works and how chemical reactions occur, and their mechanism.
2. Then break down formulas into chapters or topics.
3. To learn these formulas easily, try to make a formula notebook.
4. Sometimes students must try to make Mnemonics and short tricks, as it helps in quick revision.
5. Try to solve as many questions and revise
6. Try to use diagrams and flowcharts.
Frequently Asked Questions (FAQs)
Chemistry has three subparts (Physical, inorganic and organic). Physical chemistry has many numericals based on different formulas.
Yes, Class 12 syllabus carries more than 50% of weightage, so it is important for JEE Main Exam.
JEE Main is an exam conducted for those aspirants who want to take admission in NIITs, IITs and other engineering colleges, and it is also an eligibility test for JEE advance.
On Question asked by student community
Hi Student
If you have scored 83.4 per cent in the JEE Main examination , you can get admission into Amrita Vishwa Vidyapeetham. You need to check your eligibility before you proceed with your admission.
JEE Main paper Hindi mae hai. Ye link se dekhsakte hai ap.
https://engineering.careers360.com/hi/articles/jee-main-2026-question-paper
If you consider yourself an average student, you can start with D.C. Pandey (Arihant) as it explains concepts in a simpler way and helps build strong basics for JEE.
Cengage by B.M. Sharma is more detailed and has tougher problems, which are useful for higher-level practice. You can begin with
Hi,
Yes you can apply for BTech Mechanical Engineering with Class 12 marks through PMSSS scholarship. Here are some of the colleges accepting admissions for BTech with Class 12 marks .
Usha Mittal of Technology has no AI quota officially. please contact the college for any mangement quota seats.
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
Last Date to Apply: 15th March | NAAC A++ Accredited | Accorded institution of Eminence by Govt. of India | NIRF Rank #3
70th University Ranked by NIRF | 80th Engineering Rank by NIRF | Accredited by NBA and NAAC A+
Last Date to Apply: 26th March | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
Highest CTC 44.14 LPA | UGC Approved | 1600+ Recruiters | 100% Placement