Amity University-Noida B.Tech Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Top 10 Most Repeated Topics In Chemistry For JEE Main - Chemistry is often seen as the most scoring section in JEE Main, as it requires less time to solve compared to Physics and Mathematics. With the right preparation, students can secure strong marks by focusing on the areas that carry the highest weightage. Over the years, certain chapters in Chemistry have been repeated more frequently in JEE Main, making them important for every aspirant to prioritize during their revision. JEE Main 2026 January Session was conducted successfully, and the results for the session have also been announced. The upcoming JEE Main 2026 April Session will be conducted from 02 April to 09 April.
Candidates are advised to make changes or correct their JEE Mains 2026 form before the deadline. The authority will close the JEE Mains 2026 Correction Window today, i.e., February 28, 2026, at 11:50 PM.
This Story also Contains

The JEE Main chemistry is known for being the most scoring subject so far. Hence, in this article, we will help you prepare for chemistry by looking at the most repeated chemistry topics for the JEE Main April Session. Not only that, but we will also be delving into the top 10 most repeated topics in chemistry for JEE Main to help you get exam-ready. First, let's start with understanding the exam pattern and getting familiar with the details of JEE Main 2026.
Download Ebooks PDFs:
Preparing for any exam requires research, and especially for a competitive entrance exam like JEE Main, requires thorough preparation. So, our experts have collected and done thorough research from the JEE Main Latest Syllabus 2026. In the table below, we have provided you with the top 10 most repeated topics in chemistry for JEE Main 2026. This data comprises the last 10 years of JEE Main question papers of all slots.
This table contains the most repeated chemistry topics for JEE Main. For more details, we have also provided you with a count of the total number of questions from these topics, along with the chapters that they are from.
Chapter | Topic | Number of Questions |
Some Basic Concepts of Chemistry | Concentration Terms | 48 |
Some Basic Concepts of Chemistry | Mole Concept and Molar Mass | 48 |
Some Basic Concepts of Chemistry | Stoichiometry, Stoichiometric Calculations and Limiting Reagent | 39 |
Organic Compounds containing Oxygen | Reduction and Oxidation Reaction | 37 |
Organic Chemistry – General Organic Chemistry + Hydrocarbons | Stereoisomerism | 34 |
Biomolecules | Disaccharides and Polysaccharides | 33 |
Chemical Kinetics | 33 | |
Redox Reactions/d-Block Elements | Oxidation State | 32 |
As seen, the topic Concentration Terms happens to be the most important with the highest weighted topic. The 10th most important topic is Oxidation State, a part of both the chapters: d-Block Element/Redox Reactions. Following this list, we have more highly weighted topics that could be a game-changer for the JEE Main chemistry exam.
Now, after seeing the topics, let’s dive into the most important chapters for JEE Main Chemistry 2026. Understanding this aspect will give you an upper hand in the preparation for the exam!
We have already seen the most repeated chemistry topics for JEE Main and the chapters that they are asked from. Now, let’s see which chapters have the highest number of questions from the past 10 years. The most important chapters for JEE Main Chemistry 2026 are curated by analyzing the frequency of the questions asked from the whole chapter in the last 10 years.
This table contains the chapter name on one side and the total number of questions asked from each chapter on the other side. The following listed chapters constitute the most important chapters for chemistry, with organic compounds containing oxygen being the highest weighted chapter. This list has the most important chapters ranked in order of most to least important.
Chapter | No. of Questions (Last 10 Years) |
Organic Compounds containing Oxygen | 259 |
Co-ordination Compounds | 185 |
188 | |
Redox Reaction and Electrochemistry | 173 |
Organic Compounds Containing Nitrogen | 172 |
Chemical Thermodynamics | 166 |
Atomic Structure | 155 |
Chemical Bonding and Molecular Structure | 151 |
149 | |
d - and f - BLOCK ELEMENTS | 148 |
Some basic concepts in chemistry | 142 |
136 | |
Some Basic Principles of Organic Chemistry | 136 |
120 |
Refer to JEE Main Chapter-Wise Weightage for a better understanding of important topics and help you to prepare accordingly.
In this section, we will be diving into each topic and looking at the set of the most important questions from those particular topics listed above individually. Understanding the level of these questions and practicing them will help you increase your chances of scoring better and increasing your rank in the JEE Main exam 2026. Let’s dive in!
Question: Some amount of dichloromethane $\left(\mathrm{CH}_2 \mathrm{Cl}_2\right.$) is added to 671.141 mL of chloroform $\left(\mathrm{CHCl}_3\right)$ to prepare $2.6 \times 10^{-3} \mathrm{M}$ solution of $\mathrm{CH}_2 \mathrm{Cl}_2(\mathrm{DCM})$. The concentration of DCM is ppm (by mass).
Given : atomic mass : $\mathrm{C}=12$
$\begin{aligned} & \mathrm{H}=1 \\ & \mathrm{Cl}=35.5\end{aligned}$
density of $\mathrm{CHCl}_3=1.49 \mathrm{~g} \mathrm{~cm}^{-3}$
Solution:
Molar mass $=12+2+71$
= 85mmoles of DCM $=671.141 \times 2.6 \times 10^{-3}$
mass of solution $=1.49 \times 671.141$
$\mathrm{PPM}=\frac{671.141 \times 2.6 \times 10^{-3} \times 85 \times 10^{-3}}{1.49 \times 671.141} \times 10^6$
$=148.322$..
Hence, the answer is (148.322).
Question: The molarity of 0.006 moles of NaCl in 100 ml solutions in -
(1) 0.6
(2) 0.06
(3) 0.006
(4) 0.066
Solution:
As we learn
Molarity -
$\begin{aligned} & \text { Molarity }=\frac{\text { Moles of solute }}{\text { Vol.of solution }(L)} \\ & \text { Molarity }=\frac{\text { Moles of solute }}{\text { Vol.of solution }(L)} \\ & M=\frac{n}{V(l)}=\frac{0.006}{0.1}=0.06\end{aligned}$
Hence, the answer is the option (2).
Question: 1 gram of a carbonate ($\mathrm{M}_2 \mathrm{CO}_3$) on treatment with excess HCl produces 0.01186 mole of $\mathrm{CO}_2$.. The molar mass of $\mathrm{M}_2 \mathrm{CO}_3$ in $\mathrm{g} \mathrm{mol}^{-1}$ is :
(1) 118.6
(2) 11.86
(3) 1186
(4) 84.3
Solution:
Given,
Mass of carbonate ($\left.\mathrm{M}_2 \mathrm{CO}_3\right)$ = 1 gram
As we have learned,
Number of Moles -
No of moles = given mass of substance/ molar mass of a substance
Given the Chemical reaction,
$\mathrm{M}_2 \mathrm{CO}_3+2 \mathrm{HCl} \rightarrow 2 \mathrm{MCl}+2 \mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
From the balanced equation
$\frac{1}{M}=0.01186 \Rightarrow M=\frac{1}{0.01186}=84.3$
Hence, the answer is an option (4).
Magnetic moment explains the magnetic behavior of coordination compounds using Valence Bond Theory (VBT). Questions are mostly conceptual and numerical, moderate in difficulty.
Question: The calculated magnetic moments (spin only value) for species $\left[\mathrm{FeCl}_4\right]^{2-},\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}$ and $\mathrm{MnO}_4^{2-}$ respectively are :
(1) 5.82, 0 and $0 B M$
(2) $5.92,4.90$ and $0 B M$
(3) $4.90,0$ and $1.73 B M$
(4) $4.90,0$ and $2.83 B M$
Solution:
$\left[\mathrm{FeCl}_4\right]^{2-} \quad \mathrm{Fe}^{2+} \quad 3 \mathrm{~d}^6 \rightarrow 4$ unpaired electron
as Cl– in a weak field liquid.
$\mu_{\text {spin }}=\sqrt{24} 8 \mathrm{M}=4.9 \mathrm{BM}$
$\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^3 \quad \mathrm{Co}^{3+} \quad 3 \mathrm{~d}^6 \rightarrow$ for $\mathrm{Co}^{3+}$ with coodination no. 6
$\mathrm{C}_2 \mathrm{O}_4^{2-}$ is strong field ligand & causes pairing & hence no. unpaired electron.
$\mu_{\text {spin }}=0$
$\left[\mathrm{MnO}_4\right]^{2-} \quad \mathrm{Mn}^{+6} \quad$ it has one unpaired electron.
$\mu_{\text {spin }}=\sqrt{3} \mathrm{BM}=1.73 \mathrm{BM}$
Hence,the answer is the option(3).
Question: A sample of $\mathrm{NaClO}_3$ is converted by heat to NaCl with a loss of 0.16 g of oxygen. The residue is dissolved in water and precipitated as AgCl. The mass of AgCl (in g) obtained will be:
(1) 0.35
(2) 0.41
(3) 0.48
(4) 0.54
Solution:
As we learnt in
Stoichiometry -
Stoichiometry deals with measurements of reactants and products in a chemical reaction.
- wherein
$\mathrm{aA}(\mathrm{g})+\mathrm{bB}(\mathrm{g}) \rightarrow \mathrm{cC}(\mathrm{g})+\mathrm{dD}(\mathrm{g})$
Here, ‘a’ moles of A(g) reacts with ‘b’ moles of B(g) to give ‘c’ mole of C(g) and ‘d’ moles of D(g)
$2 \mathrm{NaClO}_3 \xrightarrow{\Delta} 2 \mathrm{NaCl}+\underset{0.16 \mathrm{~g}}{3 \mathrm{O}_2}$
$\frac{n_{\mathrm{NaCl}}}{2}=\frac{n_{\mathrm{O}_2}}{3}$
$n_{\mathrm{NaCl}}=\frac{0.16}{32} \times \frac{2}{3}=\frac{1}{200} \times \frac{2}{3}=\frac{1}{300}$
$\mathrm{NaCl} \rightarrow \mathrm{AgCl}$
PoAC on Cl
$1 \times n_{\mathrm{NaCl}}=1 \times n_{\mathrm{AgCl}}$
$\frac{1}{300}=n_{A g C l}$
the weight of $A g C l=\frac{1}{300} \times[108+35.5]=\frac{1}{300} \times 143.5=0.48 g$
Hence, the answer is an option (3).
CFT explains color, magnetism, and stability of coordination compounds. Questions are mostly conceptual and numerical, moderate in difficulty.
Question: Which of the following 3 d -metal ion will give the lowest enthalpy of hydration $\left(\Delta_{\text {hyd }} \mathrm{H}\right)$ when dissolved in water?
(1) $\mathrm{Cr}^{2+}$
(2) $\mathrm{Mn}^{2+}$
(3) $\mathrm{Fe}^{2+}$
(4) $\mathrm{Co}^{2+}$
Solution:
Water act as a weak ligand.C F S E of metal ions depend on strength of ligand.
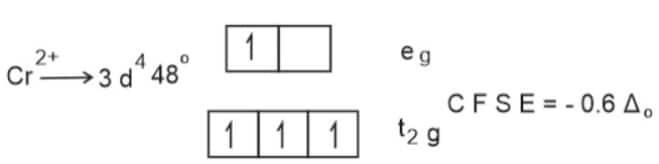
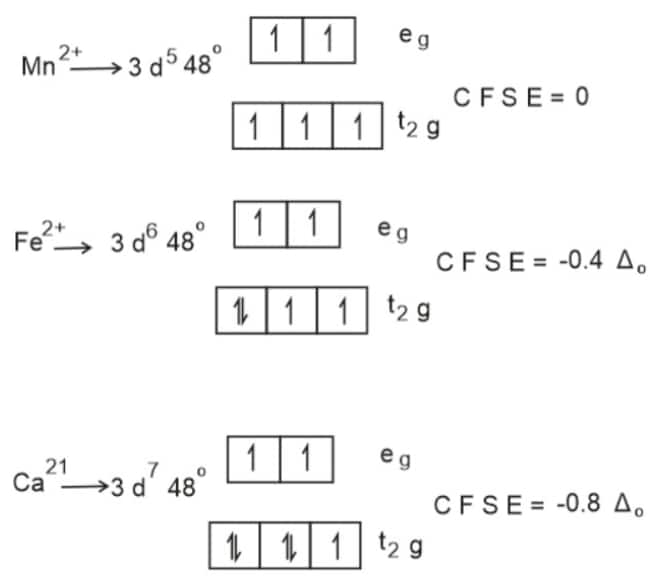
Since the CFSE of $\mathrm{Mn}^{2+}$ is the least, $\Delta \mathrm{H}_{\mathrm{Hyd}}$ of it is also lowest.
Hence, the answer is the option (2).
Redox reactions are key in electrochemistry and organic transformations. Questions are mostly numerical and moderate in difficulty.
Question: Experimentally reducing a functional group cannot be done by which one of the following reagents?
(1) $\mathrm{Zn} / \mathrm{H}_2 \mathrm{O}$
(2) $\mathrm{Pt}-\mathrm{C} / \mathrm{H}_2$
(3) $\mathrm{Pd}-\mathrm{C} / \mathrm{H}_2$
(4) $\mathrm{Na} / \mathrm{H}_2$
Solution:
Out of the given reagents, $\mathrm{Na} / \mathrm{H}_2$ is not used as a reducing agent.
Hence, the answer is the option (4).
Stereoisomerism explains spatial arrangement of atoms in molecules, important in organic chemistry. Questions are mostly conceptual and moderate in difficulty.
Question: The total number of possible isomers for square-planar $\left[\mathrm{Pt}(\mathrm{Cl})\left(\mathrm{NO}_2\right)\left(\mathrm{NO}_3\right)(\mathrm{SCN})\right]^{2-}$ is :
(1) 8
(2) 12
(3) 16
(4) 24
Solution:
As we have learnt,
$\mathrm{NO}_2^{-}$ and $S C N^{-}$ are ambidentate ligands and each of them can attach through two different donor sites.
Now, the given square planar complex can show geometrical isomerism as well as linkage isomers.
The number of isomers possible is listed below:
| Compound | Number of Isomers |
| $\left[\mathrm{Pt}(\mathrm{Cl})\left(\mathrm{NO}_2\right)\left(\mathrm{NO}_3\right)(\mathrm{SCN})\right]^{2-}$ | 3 |
| $\left[\mathrm{Pt}(\mathrm{Cl})(\mathrm{ONO})\left(\mathrm{NO}_3\right)(\mathrm{SCN})\right]^{2-}$ | 3 |
| $\left[\mathrm{Pt}(\mathrm{Cl})(\mathrm{ONO})\left(\mathrm{NO}_3\right)(\mathrm{NCS})\right]^{2-}$ | 3 |
| $\left[\mathrm{Pt}(\mathrm{Cl})\left(\mathrm{NO}_2\right)\left(\mathrm{NO}_3\right)(\mathrm{NCS})\right]^{2-}$ | 3 |
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
Thus, the total number of Isomers is 12.
Hence, the answer is an option (2).
This topic is important in biomolecules, especially for carbohydrates' structure and properties. Questions are mostly conceptual and numerical, moderate in difficulty.
Question: Compound A gives D-Galactose and D-Glucose on hydrolysis. The compound A is :
(1) Amylose
(2) Sucrose
(3) Maltose
(4) Lactose
Solution:
As we have learned,
The monosaccharides are involved in the formation of the given sugars.
Amylose: $\alpha-\mathrm{D}$ Glucose
Sucrose : $\alpha-$ D-Glucose $+\beta-$ D-Fructose
Maltose : $\alpha$ - D-Glucose
Lactose : $\beta$ - D-Galactose $+\beta$ - D-Glucose
Hence, the answer is the option (4).
First-order reactions are important in chemical kinetics. Questions are mostly numerical and moderate in difficulty.
Question: For a first-order reaction, $\mathrm{A} \rightarrow \mathrm{P}, \mathrm{t}_{\frac{1}{2}}$ , (half-life) is 10 days. The time required for $\frac{1}{4}^{\text {th }}$conversion of A (in days) is :
(ln 2=0.693, ln 3=1.1)
(1) 4.1
(2) 3.2
(3) 5
(4) 2.5
Solution:
For the first-order reaction: -
$\begin{aligned} & K=\frac{0.693}{t_{\frac{1}{2}}}=\frac{0.693}{10} \\ & K=\frac{2.303}{t} \log \frac{R_o}{R_t} \\ & \frac{0.693}{10}=\frac{2.303}{t} \log \frac{R_o \times 4}{3 R_0} \\ & \frac{0.693}{10}=\frac{2.303}{t}[\log 4-\log 3]=\frac{2.303}{t}[0.6020-0.4771] \\ & \frac{0.693}{10}=\frac{2.303}{t} \times 0.1249 \\ & t=\frac{2.303 \times 0.1249 \times 10}{0.603}=4.15 \text { days }\end{aligned}$
Hence, the correct answer is option (1)
Question: The amphoteric oxide among $\mathrm{V}_2 \mathrm{O}_3, \mathrm{~V}_2 \mathrm{O}_4$ and $\mathrm{V}_2 \mathrm{O}_5$ upon reaction with alkali leads to formation of an oxide anion. The oxidation state of V in the oxide anion is:
(1) +3
(2) +7
(3) +5
(4) +4
Solution:
$\mathrm{V}_2 \mathrm{O}_3$ - basic
$\mathrm{V}_2 \mathrm{O}_4$ - weakly acidic or amphoteric
$\mathrm{V}_2 \mathrm{O}_5$ - amphoteric
$
\mathrm{V}_2 \mathrm{O}_5+\text { alkali } \rightarrow \mathrm{VO}_4^{3-}
$
In $\mathrm{VO}_4^{3-}$ ion, vanadium is in a +5 oxidation state.
Hence, the correct answer is option (3).
Students can follow JEE Main- Top 30 Most Repeated Questions & Topics for better practice and revision. Along with that, try to solve the JEE Main 2026 Sample Paper.
Chemistry is divided into three branches: Physical, Organic, and Inorganic Chemistry. And it is one of the most important and scoring subjects if you are preparing for JEE Main 2026. To study chemistry in a better way, students must study each branch separately, focusing on its core concepts, formulas, and reaction mechanisms.
It is important to learn important reactions, formulas, and periodic table trends.
Read the NCERT textbook and solve exercises
Revise key topics frequently and solve full-length mock tests
Solve previous year questions
For the complete preparation strategy, follow the JEE Main 2026 complete preparation strategy
Frequently Asked Questions (FAQs)
The most repeated topics include Concentration Terms (Solutions), Mole Concept, Redox Reactions, Stoichiometry, Coordination Compounds, Chemical Kinetics, Chemical Bonding, and Oxidation States. These topics consistently appear in JEE Mains over the years.
Actually, no! JEE Mains is a national competitive exam and students should refer to a lot of other books as well for the in depth preparation of chemistry. So you must refer to 2-3 books outside NCERT as well!
Inorganic chemistry has the easiest syllabus and is the highest to score. It is advisable to not leave any topic of this part of chemistry if you’re planning to bag a good rank in the exam.
Start with high-weightage and repeated topics like Mole Concept, Thermodynamics, Coordination Compounds, Redox Reactions, and Hydrocarbons. Once strong in these, cover remaining chapters systematically to ensure no topic is left uncovered.
On Question asked by student community
decent chances actually as home state quota seats are 50%. allotments will depend on the JEE rank and not percentile though. in 2025, for female supernumerary it closed at 9286 rank while for open gen it closed at 5573.
So, would advise to use this tool to check the probable
yes you will. Ususally the return is within 7 days i it has failed at the gateway level which it seems to be. Please wait. You will get the money back
Slim chances as in 2025 the closing rank was 118 for SPA Delhi for B.Arch. You will need to wait for the rank list to come in April before getting a better picture. Please check https://engineering.careers360.com/jee-main-college-predictor for the predictions.
Check out https://engineering.careers360.com/jee-main-rank-predictor to know the probable rank
Hey Abhinav!
You can start your JEE Preparation with ICSE. You can check How to Prepare for JEE Main 2026?- Study Plan and start you preparation.
An 87.12% in JEE is good. To get admission to VIT, you'll need to take the VITEEE. You can apply for VITEEE if you meet the eligibility criteria for class 12. Your JEE score is not required for this process. Admission to VIT will be based on your VITEEE score.
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
70th University Ranked by NIRF | 80th Engineering Rank by NIRF | Accredited by NBA and NAAC A+
Last Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
Highest CTC 44.14 LPA | UGC Approved | 1600+ Recruiters | 100% Placement
NAAC A++ Grade | Recognized as Category-1 Deemed to be University by UGC | 41,000 + Alumni Imprints Globally