Amity University-Noida B.Tech Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
जेईई मेन्स 2026 के लिए रसायन विज्ञान में टॉप 10 सबसे अधिक दोहराए जाने वाले विषय- जेईई मेन में रसायन विज्ञान को अक्सर सबसे अधिक स्कोरिंग सेक्शन के रूप में देखा जाता है, क्योंकि इसे हल करने में भौतिकी और गणित की तुलना में कम समय लगता है। सही तैयारी के साथ, छात्र सबसे अधिक महत्व वाले क्षेत्रों पर ध्यान केंद्रित करके अच्छे अंक प्राप्त कर सकते हैं। पिछले कुछ वर्षों में, रसायन विज्ञान के कुछ अध्यायों को जेईई मेन में बार-बार दोहराया गया है, जिससे प्रत्येक अभ्यर्थी के लिए अपने रिविज़न के दौरान प्राथमिकता देना महत्वपूर्ण हो गया है। प्राधिकरण द्वारा जेईई मेन 2026 आवेदन 31 अक्टूबर को जारी कर दिए गए है। उम्मीदवार 27 नवंबर तक जेईई मेन 2026 के लिए आवेदन कर सकते हैं।
आधिकारिक जेईई मेन 2026 सिलेबस देखें

जेईई मेन्स परीक्षा में रसायन विज्ञान को अब तक का सबसे अधिक स्कोरिंग विषय माना जाता है। इसलिए, इस लेख में हम जेईई मेन्स परीक्षा में बार-बार दोहराए जाने वाले रसायन विज्ञान विषयों पर ध्यान केंद्रित करके आपको रसायन विज्ञान की तैयारी में मदद करेंगे। इसके साथ ही हम आपको परीक्षा की तैयारी में मदद करने के लिए जेईई मेन्स परीक्षा में रसायन विज्ञान में टॉप 10 सबसे अधिक दोहराए जाने वाले विषयों पर भी चर्चा करेंगे। सबसे पहले, आइए परीक्षा पैटर्न को समझें और जेईई मेन्स 2026 के विवरण से परिचित हों।
किसी भी परीक्षा की तैयारी के लिए शोध की आवश्यकता होती है और विशेष रूप से जेईई मेन्स जैसी प्रतियोगी प्रवेश परीक्षा के लिए गहन तैयारी की आवश्यकता होती है। इसलिए, हमारे विशेषज्ञों ने नवीनतम जेईई मेन सिलेबस 2026 से गहन शोध एकत्र किया है। नीचे दी गई तालिका में हमने आपको जेईई मेन्स 2026 के लिए रसायन विज्ञान में टॉप 10 सबसे अधिक दोहराए गए विषय प्रदान किए हैं। इस डेटा में सभी स्लॉट के पिछले 10 वर्षों के जेईई मेन्स प्रश्न पत्र शामिल हैं।
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
इस तालिका में जेईई मेन्स के लिए सबसे अधिक दोहराए गए रसायन विज्ञान विषय शामिल हैं। अधिक जानकारी के लिए हमने आपको इन विषयों से पूछे गए प्रश्नों की कुल संख्या तथा उनसे संबंधित अध्यायों की जानकारी भी उपलब्ध कराई है।
चैप्टर | टॉपिक | प्रश्नों की संख्या |
|---|---|---|
रसायन विज्ञान की मूल अवधारणाएँ | कंसंट्रेशन टर्म | 48 |
रसायन विज्ञान की मूल अवधारणाएँ | मोल अवधारणा और मोलर द्रव्यमान | 48 |
रसायन विज्ञान की मूल अवधारणाएँ | स्टोइकोमेट्री, स्टोइकोमेट्रिक गणना और सीमांत अभिकर्मक | 39 |
ऑक्सीजन युक्त कार्बनिक यौगिक | अपचयन और ऑक्सीकरण अभिक्रिया | 37 |
कार्बनिक रसायन विज्ञान - सामान्य कार्बनिक रसायन विज्ञान + हाइड्रोकार्बन | स्टीरियोआइसोमेरिज्म | 34 |
जैविक अणु | डाइसैकेराइड और पॉलीसैकेराइड | 33 |
रासायनिक गतिकी | 33 | |
रेडॉक्स अभिक्रियाएँ/डी-ब्लॉक तत्व | ऑक्सीकरण अवस्था | 32 |
जैसा कि देखा गया है, कंसंट्रेशन टर्म विषय सबसे महत्वपूर्ण है और इसका वेटेज सबसे अधिक है। 10वां सबसे महत्वपूर्ण विषय ऑक्सीकरण अवस्था है, जो दोनों अध्यायों का एक हिस्सा है: डी-ब्लॉक तत्व/रेडॉक्स अभिक्रियाएं। इस सूची के बाद, हमारे पास हाई वेटेज वाले विषयों की सूची है, जो जेईई मेन्स रसायन विज्ञान परीक्षा के लिए गेम चेंजर हो सकती हैं।
अब, टॉप 10 विषयों की सूची देखने के बाद, आइए जेईई मेन्स केमिस्ट्री 2026 के सबसे महत्वपूर्ण अध्यायों पर विचार करते हैं। इस पहलू को समझने से आपको परीक्षा की तैयारी में सहायता मिलेगी!
हम जेईई मेन्स में सबसे ज़्यादा रिपीट होने वाले केमिस्ट्री के टॉपिक्स और उनसे पूछे जाने वाले अध्यायों को पहले ही देख चुके हैं। अब, आइए देखें कि पिछले 10 सालों में किन अध्यायों से सबसे ज़्यादा सवाल पूछे गए हैं।
इस तालिका में एक ओर अध्याय का नाम तथा दूसरी ओर प्रत्येक अध्याय से पूछे गए प्रश्नों की कुल संख्या दी गई है। नीचे सूचीबद्ध ये अध्याय रसायन विज्ञान के सबसे महत्वपूर्ण अध्याय हैं, जिनमें ऑक्सीजन युक्त कार्बनिक यौगिक सबसे महत्वपूर्ण अध्याय है।
अध्याय | प्रश्नों की संख्या (पिछले 10 वर्षों में) |
|---|---|
ऑक्सीजन युक्त कार्बनिक यौगिक | 259 |
216 | |
उपसहसंयोजक यौगिक | 185 |
188 | |
रेडॉक्स अभिक्रिया और इलेक्ट्रोकेमिस्ट्री | 173 |
नाइट्रोजन युक्त कार्बनिक यौगिक | 172 |
रासायनिक ऊष्मागतिकी | 166 |
परमाणु संरचना | 155 |
रासायनिक बंधन और आणविक संरचना | 151 |
149 | |
डी - और एफ - ब्लॉक तत्व | 148 |
रसायन विज्ञान में मूल अवधारणाएँ | 142 |
136 | |
कार्बनिक रसायन विज्ञान के मूल सिद्धांत | 136 |
120 |
महत्वपूर्ण विषयों की बेहतर समझ के लिए जेईई मेन अध्याय-वार वेटेज देखें और इसके अनुसार अपनी तैयारी करें।
इस अनुभाग में, हम प्रत्येक विषय पर गहनता से विचार करेंगे तथा ऊपर सूचीबद्ध विशेष विषयों से सबसे महत्वपूर्ण प्रश्नों के समूह पर अलग-अलग चर्चा करेंगे। इन प्रश्नों के स्तर को समझने और उनका अभ्यास करने से आपको बेहतर स्कोर करने और जेईई मेन परीक्षा 2026 में बेहतर रैंक प्राप्त करने में मदद मिलेगी। आइए शुरू करते हैं!
1. कंसंट्रेशन टर्म
Question: Some amount of dichloromethane
(CH2Cl2
) is added to 671.141 mL of chloroform
(CHCl3)
to prepare
2.6×10−3M
solution of
CH2Cl2(DCM)
. The concentration of DCM is ppm (by mass).
Given : atomic mass :
C=12
H=1Cl=35.5
density of
CHCl3=1.49 g cm−3
Solution:
Molar mass
=12+2+71
= 85mmoles of DCM
=671.141×2.6×10−3
mass of solution
=1.49×671.141
PPM=671.141×2.6×10−3×85×10−31.49×671.141×106
=148.322
..
Hence, the answer is (148.322).
Question: The molarity of 0.006 moles of NaCl in 100 ml solutions in -
(1) 0.6
(2) 0.06
(3) 0.006
(4) 0.066
Solution:
As we learn
Molarity -
Molarity = Moles of solute Vol.of solution (L) Molarity = Moles of solute Vol.of solution (L)M=nV(l)=0.0060.1=0.06
Hence, the answer is the option (2).
2. मोल अवधारणा और मोलर द्रव्यमान
Question: 1 gram of a carbonate (
M2CO3
) on treatment with excess HCl produces 0.01186 mole of
CO2
.. The molar mass of
M2CO3
in
gmol−1
is :
(1) 118.6
(2) 11.86
(3) 1186
(4) 84.3
Solution:
Given,
Mass of carbonate (
M2CO3)
= 1 gram
As we have learned,
Number of Moles -
No of moles = given mass of substance/ molar mass of a substance
Given the Chemical reaction,
M2CO3+2HCl→2MCl+2H2O+CO2
From the balanced equation
1M=0.01186⇒M=10.01186=84.3
Hence, the answer is an option (4).
3. चुंबकीय आघूर्ण (वीबीटी के आधार पर)
Magnetic moment explains the magnetic behavior of coordination compounds using Valence Bond Theory (VBT). Questions are mostly conceptual and numerical, moderate in difficulty.
Question: The calculated magnetic moments (spin only value) for species
[FeCl4]2−,[Co(C2O4)3]3−
and
MnO42−
respectively are :
(1) 5.82, 0 and
0BM
(2)
5.92,4.90
and
0BM
(3)
4.90,0
and
1.73BM
(4)
4.90,0
and
2.83BM
Solution:
[FeCl4]2−Fe2+3 d6→4
unpaired electron
as Cl– in a weak field liquid.
μspin =248M=4.9BM
[Co(C2O4)3]3Co3+3 d6→
for
Co3+
with coodination no. 6
C2O42−
is strong field ligand & causes pairing & hence no. unpaired electron.
μspin =0
[MnO4]2−Mn+6
it has one unpaired electron.
μspin =3BM=1.73BM
Hence,the answer is the option(3).
4. स्टोइकोमेट्री, स्टोइकोमेट्रिक गणना और सीमांत अभिकर्मक
Question: A sample of
NaClO3
is converted by heat to NaCl with a loss of 0.16 g of oxygen. The residue is dissolved in water and precipitated as AgCl. The mass of AgCl (in g) obtained will be:
(1) 0.35
(2) 0.41
(3) 0.48
(4) 0.54
Solution:
As we learnt in
Stoichiometry -
Stoichiometry deals with measurements of reactants and products in a chemical reaction.
- wherein
aA(g)+bB(g)→cC(g)+dD(g)
Here, ‘a’ moles of A(g) reacts with ‘b’ moles of B(g) to give ‘c’ mole of C(g) and ‘d’ moles of D(g)
2NaClO3→Δ2NaCl+3O20.16 g
nNaCl2=nO23
nNaCl=0.1632×23=1200×23=1300
NaCl→AgCl
PoAC on Cl
1×nNaCl=1×nAgCl
1300=nAgCl
the weight of
AgCl=1300×[108+35.5]=1300×143.5=0.48g
Hence, the answer is an option (3).
5. सीएफटी (क्रिस्टल फील्ड थ्योरी) के अनुप्रयोग
CFT explains color, magnetism, and stability of coordination compounds. Questions are mostly conceptual and numerical, moderate in difficulty.
Question: Which of the following 3 d -metal ion will give the lowest enthalpy of hydration
(Δhyd H)
when dissolved in water?
(1)
Cr2+
(2)
Mn2+
(3)
Fe2+
(4)
Co2+
Solution:
Water act as a weak ligand.C F S E of metal ions depend on strength of ligand.
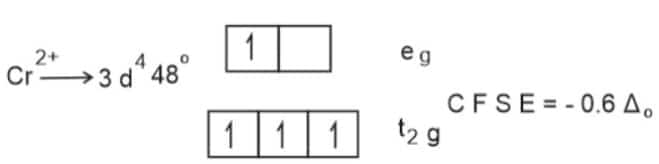
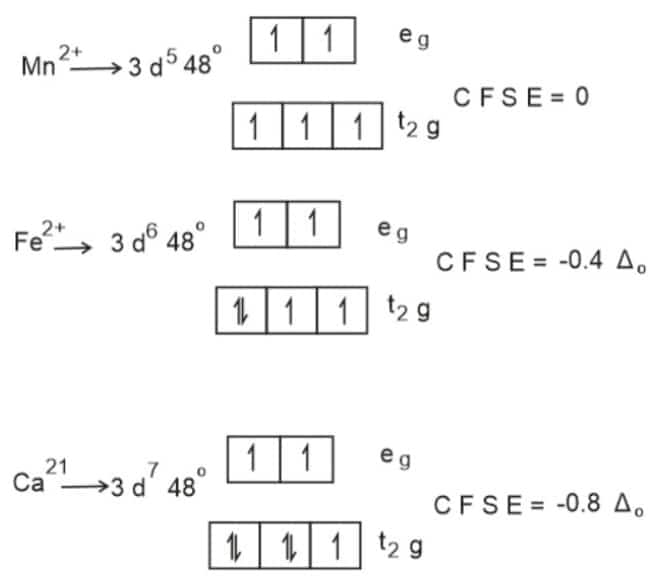
Since the CFSE of
Mn2+
is the least,
ΔHHyd
of it is also lowest.
Hence, the answer is the option (2).
6. अपचयन और ऑक्सीकरण अभिक्रिया
Redox reactions are key in electrochemistry and organic transformations. Questions are mostly numerical and moderate in difficulty.
Question: Experimentally reducing a functional group cannot be done by which one of the following reagents?
(1)
Zn/H2O
(2)
Pt−C/H2
(3)
Pd−C/H2
(4)
Na/H2
Solution:
Out of the given reagents,
Na/H2
is not used as a reducing agent.
Hence, the answer is the option (4).
7. स्टीरियोआइसोमेरिज्म
Stereoisomerism explains spatial arrangement of atoms in molecules, important in organic chemistry. Questions are mostly conceptual and moderate in difficulty.
Question: The total number of possible isomers for square-planar
[Pt(Cl)(NO2)(NO3)(SCN)]2−
is :
(1) 8
(2) 12
(3) 16
(4) 24
Solution:
As we have learnt,
NO2−
and
SCN−
are ambidentate ligands and each of them can attach through two different donor sites.
Now, the given square planar complex can show geometrical isomerism as well as linkage isomers.
The number of isomers possible is listed below:
Compound | Number of Isomers |
[Pt(Cl)(NO2)(NO3)(SCN)]2− | 3 |
[Pt(Cl)(ONO)(NO3)(SCN)]2− | 3 |
[Pt(Cl)(ONO)(NO3)(NCS)]2− | 3 |
[Pt(Cl)(NO2)(NO3)(NCS)]2− | 3 |
Thus, the total number of Isomers is 12.
Hence, the answer is an option (2).
8. डाइसैकेराइड और पॉलीसैकेराइड
This topic is important in biomolecules, especially for carbohydrates' structure and properties. Questions are mostly conceptual and numerical, moderate in difficulty.
Question: Compound A gives D-Galactose and D-Glucose on hydrolysis. The compound A is :
(1) Amylose
(2) Sucrose
(3) Maltose
(4) Lactose
Solution:
As we have learned,
The monosaccharides are involved in the formation of the given sugars.
Amylose:
α−D
Glucose
Sucrose :
α−
D-Glucose
+β−
D-Fructose
Maltose :
α
- D-Glucose
Lactose :
β
- D-Galactose
+β
- D-Glucose
Hence, the answer is the option (4).
9. प्रथम क्रम अभिक्रिया
First-order reactions are important in chemical kinetics. Questions are mostly numerical and moderate in difficulty.
Question: For a first-order reaction,
A→P,t12
, (half-life) is 10 days. The time required for
14th
conversion of A (in days) is :
(ln 2=0.693, ln 3=1.1)
(1) 4.1
(2) 3.2
(3) 5
(4) 2.5
Solution:
For the first-order reaction: -
K=0.693t12=0.69310K=2.303tlogRoRt0.69310=2.303tlogRo×43R00.69310=2.303t[log4−log3]=2.303t[0.6020−0.4771]0.69310=2.303t×0.1249t=2.303×0.1249×100.603=4.15 days
Hence, the correct answer is option (1)
10. ऑक्सीकरण अवस्था
Question: The amphoteric oxide among
V2O3, V2O4
and
V2O5
upon reaction with alkali leads to formation of an oxide anion. The oxidation state of V in the oxide anion is:
(1) +3
(2) +7
(3) +5
(4) +4
Solution:
V2O3
- basic
V2O4
- weakly acidic or amphoteric
V2O5
- amphoteric
V2O5+ alkali →VO43−
In
VO43−
ion, vanadium is in a +5 oxidation state.
Hence, the correct answer is option (3).
बेहतर अभ्यास और रिवीजन के लिए छात्र जेईई मेन के टॉप 30 सबसे ज़्यादा बार पूछे जाने वाले प्रश्नों और विषयों को देख सकते हैं। साथ ही, जेईई मेन 2026 के सैंपल पेपर को हल करने का प्रयास करें।
रसायन विज्ञान को तीन शाखाओं में विभाजित किया गया है: भौतिक, कार्बनिक और अकार्बनिक रसायन विज्ञान। यदि आप जेईई मेन्स 2026 की तैयारी कर रहे हैं तो यह सबसे महत्वपूर्ण और स्कोरिंग विषयों में से एक है। रसायन विज्ञान का बेहतर अध्ययन करने के लिए छात्रों को प्रत्येक शाखा का अलग-अलग अध्ययन करना चाहिए, तथा उसकी मूल अवधारणाओं, सूत्रों और अभिक्रिया तंत्र पर ध्यान केंद्रित करना चाहिए।
महत्वपूर्ण अभिक्रियाओं, सूत्रों और आवर्त सारणी के ट्रेंड को सीखना महत्वपूर्ण है।
एनसीईआरटी की पाठ्यपुस्तक पढ़ें और अभ्यास करें
मुख्य विषयों को बार-बार दोहराएँ और पूर्ण-लंबाई वाले मॉक टेस्ट हल करें
पिछले वर्ष के प्रश्न हल करें
जेईई मेन 2026 की पूरी तैयारी रणनीति का पालन करें
Frequently Asked Questions (FAQs)
मोल अवधारणा, ऊष्मागतिकी, उपसहसंयोजन यौगिक, रेडॉक्स अभिक्रियाएँ और हाइड्रोकार्बन जैसे हाई वेटेज वाले विषयों से शुरुआत करें। एक बार इनमें अच्छी पकड़ हो जाने पर, शेष अध्यायों को व्यवस्थित रूप से पूरा करें ताकि कोई भी विषय छूट न जाए।
अकार्बनिक रसायन विज्ञान का पाठ्यक्रम सबसे आसान है और इसमें सबसे ज़्यादा अंक प्राप्त करने की संभावना है। अगर आप रसायन विज्ञान में अच्छी रैंक हासिल करना चाहते हैं, तो सलाह दी जाती है कि रसायन विज्ञान के इस भाग का कोई भी विषय न छोड़ें।
दरअसल, नहीं! जेईई मेन्स एक राष्ट्रीय प्रतियोगी परीक्षा है और छात्र रसायन विज्ञान की गहन तैयारी के लिए कई अन्य पुस्तकों का भी सहारा लेते है। इसलिए आपको एनसीईआरटी के अलावा भी 2-3 पुस्तकों का सहारा लेना चाहिए!
सबसे ज़्यादा दोहराए जाने वाले विषयों में कंसंट्रेशन टर्म (विलयन), मोल अवधारणा, रेडॉक्स अभिक्रियाएँ, स्टोइकोमेट्री, उपसहसंयोजक यौगिक, रासायनिक गतिकी, रासायनिक आबंध और ऑक्सीकरण अवस्थाएँ शामिल हैं। ये विषय वर्षों से जेईई मेन्स में लगातार आते रहे हैं।
On Question asked by student community
decent chances actually as home state quota seats are 50%. allotments will depend on the JEE rank and not percentile though. in 2025, for female supernumerary it closed at 9286 rank while for open gen it closed at 5573.
So, would advise to use this tool to check the probable
yes you will. Ususally the return is within 7 days i it has failed at the gateway level which it seems to be. Please wait. You will get the money back
Slim chances as in 2025 the closing rank was 118 for SPA Delhi for B.Arch. You will need to wait for the rank list to come in April before getting a better picture. Please check https://engineering.careers360.com/jee-main-college-predictor for the predictions.
Check out https://engineering.careers360.com/jee-main-rank-predictor to know the probable rank
Hey Abhinav!
You can start your JEE Preparation with ICSE. You can check How to Prepare for JEE Main 2026?- Study Plan and start you preparation.
An 87.12% in JEE is good. To get admission to VIT, you'll need to take the VITEEE. You can apply for VITEEE if you meet the eligibility criteria for class 12. Your JEE score is not required for this process. Admission to VIT will be based on your VITEEE score.
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
70th University Ranked by NIRF | 80th Engineering Rank by NIRF | Accredited by NBA and NAAC A+
Last Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
Highest CTC 44.14 LPA | UGC Approved | 1600+ Recruiters | 100% Placement
NAAC A++ Grade | Recognized as Category-1 Deemed to be University by UGC | 41,000 + Alumni Imprints Globally