Amity University-Noida B.Tech Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
JEE Main 2026 Question Paper: In this article, we have updated all the JEE Main 2026 question papers. You will be able to download all the JEE Mains questions paper shift wise. Using the JEE Mains 2026 question paper can help you understand the difficulty of JEE Mains 2026 and can help you prepare for April session as well. The January session is scheduled from 21 Jan to 29 January, and every question paper is updated in the article below. JEE Mains official answer key is released by NTA. Let's check the Question Paper with the answer Key.
The Session 2 examination will be conducted from April 2 to April 9, 2026. The exam will be held in two shifts:
Morning shift: 9 am to 12 noon
Afternoon shift: 3 pm to 6 pm
This Story also Contains

JEE Main 2026 Session 1 results have been announced. And your are not sure about the counselling process, expected cut-offs, or which engineering colleges to target? Our expert team is ready to guide you every step of the way. Book your FREE counselling session by filling out the Google Form.
JEE Mains 2026 question paper has been given below for Jan 21, Jan 22, Jan 23, Jan 24 and Jan 28 both shifts. You will be able to find questions with answer Key below:
| JEE Main Official Question Paper January Session 2026 |
| JEE Main Official Answer Key January Session 2026 |
The JEE Mains 2026 Jan 28 Shift 2 was concept-driven but scoring, with Physics being formula-based, Chemistry theory-focused (more inorganic), and Maths lengthy yet manageable. You can find detailed information in JEE Main 2026 January 28 Shift 2 Question Paper pdf. You can find subject wise detailed analysis given below:
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
| Subjects | Major Topics Asked | Difficulty Level |
|---|---|---|
| Physics | Modern Physics, Ray Optics, Formula-based numerical questions | Easy |
| Chemistry | Chemical Kinetics, Ionic Equilibrium (Buffer), Coordination Compounds, Amines, Inorganic-focused theory | Moderate |
| Mathematics | Vector & 3D Geometry, Integral Calculus, Differential Equations, Probability, Conic Sections, Trigonometry, Sequences & Series, Statistics | Moderate (Lengthy) |
| Overall | Statement-based questions across subjects; Maths lengthy; Physics formula-driven | Easy |
JEE Main 28 Jan Shift 1
JEE Main 28 Jan Shift 2
Memory- Based Questions JEE Main 28 Shift 2
JEE Mains 2026 Jan 28 Shift 2 Maths Questions
Q.1 The sum of coefficients of
2.
3.
4.
Q.2 If
1. 841
2. 976
3. 984
4. 890
Q.3
1.
2.
3.
4.
Question 1:
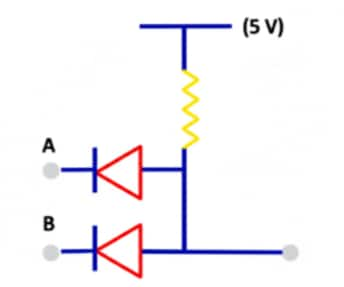
For the circuit given below, identify the logic gate.
1 AND
2 OR
3 NAND
4 NOR
Question 1: The plot of
The JEE Mains 2026 Jan 28 Shift 1 morning shift was moderate to tough. You can find detailed information in JEE Main 2026 January 28 shift 1 Question Paper For more details you can check the table below:
| Subject / Overall | Difficulty | Length | Time | Question Type | Key Point |
|---|---|---|---|---|---|
| Physics | Easy–Moderate | Short–Moderate | ~60 min | Formula + Concept | Scoring and balanced |
| Mathematics | Moderate–Difficult | Lengthy | ~70–80 min | Calculation + Concept | Toughest and most time-consuming |
| Chemistry | Moderate | Moderate–Long | ~55–60 min | Statement-based + Named reactions | Organic-heavy but manageable |
| Overall Paper | Moderate–Hard | Lengthy | Time pressure | Statement-based trend | Maths toughest; Physics easiest |
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
98% Placement Record | Highest CTC 81.25 LPA | NAAC A++ Accredited | Ranked #62 in India by NIRF Ranking 2025 | JEE & JET Scores Accepted
Memory- Based Questions JEE Main 28 Shift 1
1.
If
3
2
4
6
2.
Equation of an EMW in a medium is given by
Question 3: In Carius method of estimation of ' Br ', 1.53 g of an organic compound gave 1 g AgBr . The \% of Br in organic compound is, (Atomic mass of
1) 35.23
2) 43.53
3) 27.81
4) 22.71
Question 4: Find the ratio of de Broglie wavelength of duetron having enorgy
The JEE Mains 2026 Jan 24 Shift 1 (morning shift).The overall difficulty level of the exam was Moderate. You can check detail analysis and questions JEE Main January 24 shift 1 Question Paper
Few Memory -Based Questions
Question 1: An electron make transition from higher energy orbit (
Question 2: Which of the following has the lowest bond energy?
Question 3: Choose the correct order of second IE of O, C, N and F
Question 4:
Question 5: Which of the following molecules is secondary alcohol?
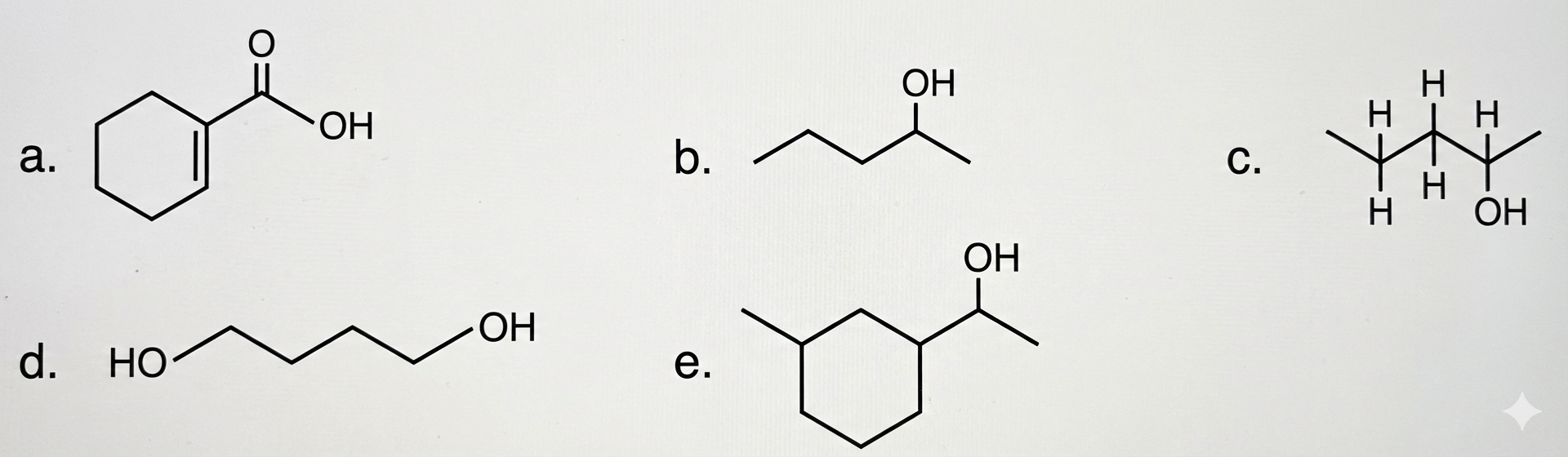
1 b, c, e only
2 b, c, d, e only
3 a, c, d, e only
4 a, b, d only
Question 4:
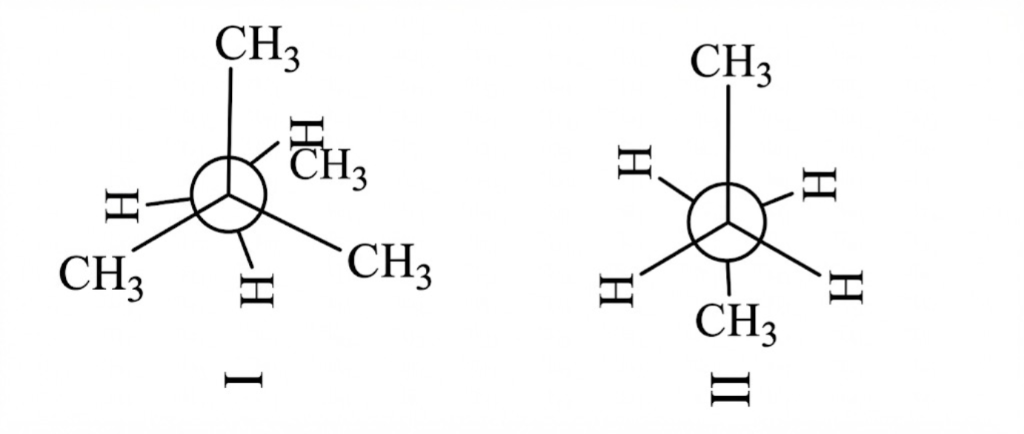
Statement I= II is more stable than (I)
Statement II=As dihedral angle increases, stability decrease
(a) I: correct, II: Incorrect
(b) I: correct, II: correct
(c)
(d)
Compared to the 24th January JEE Main 2026 morning shift, this evening shift was almost similar. In terms of difficulty, the overall order was Chemistry > Mathematics> Physics for the 24th January evening shift Question paper
Few Memory-Based Questions
Question 1: statement-1 : Two different aldehyde on cross aldol condensation always give
four product :
statement-II: Among benzaldehyde and acetophenone only acetophenone reacts with semicarbazine.
(1) Statement-I and Statement-II both are correct
(2) Statement-I is incorrect Statement-II is correct
(3) Statement-I is correct Statement-II is incorrect
(4) Statement-1 and Statement-II both incorrect
Question 2: How many linear tripeptides are possible with valine (Val), Glycine (Gly) and Alanine (Ala). No amino acid should be repeated?
1) 8
2) 5
3) 6
4) 4
Question 3: Order of wavelength of absorbed radiation for the below given complexes is,
(a)
(b)
(c)
(d)
Question 4: Given :
Find out bond energy (
Question 5: Which of the following have same bond order and are paramagnetic?
The JEE Mains 2026 Jan 23 Shift 1 (morning shift) was easy to moderate. You can also check JEE Main 2026 January 23 Shift 1 Question Paper .
| Subject | Difficulty Level | Key Observation |
|---|---|---|
| Physics | Easy | Formula-based, scoring |
| Chemistry | Moderate | Conceptual and statement-based |
| Mathematics | Moderate–Difficult | Lengthy, time-consuming calculations |
| Overall | Easy–Moderate | Comparable to JEE Main 2025 shifts |
Find some memory-based questions below:
Question 1: The correct order of ionisation energy of
Question 2: Given below are two statements
Statement-I :
Statement-II :
1 Both S-I and S-II are correct
2 Both S-I and S-II are incorrect
3 S-I is correct and S-II are incorrect
4 S-I is incorrect and S-II are correct
Question 3: A rectangle is formed by lines
Question 4: Let
Let I = number of elements in R
n= minimum number of element
be added in
1)10
2)17
3)11
4) 14
Question 5: Find out the correct energy for the grand state or energy transition. (symbols have usual meaning &
The JEE Mains 2026 Jan 23 Shift 2 (Evening shift) was moderate. You can also check JEE Main 2026 January 23 Shift 1 Question Paper
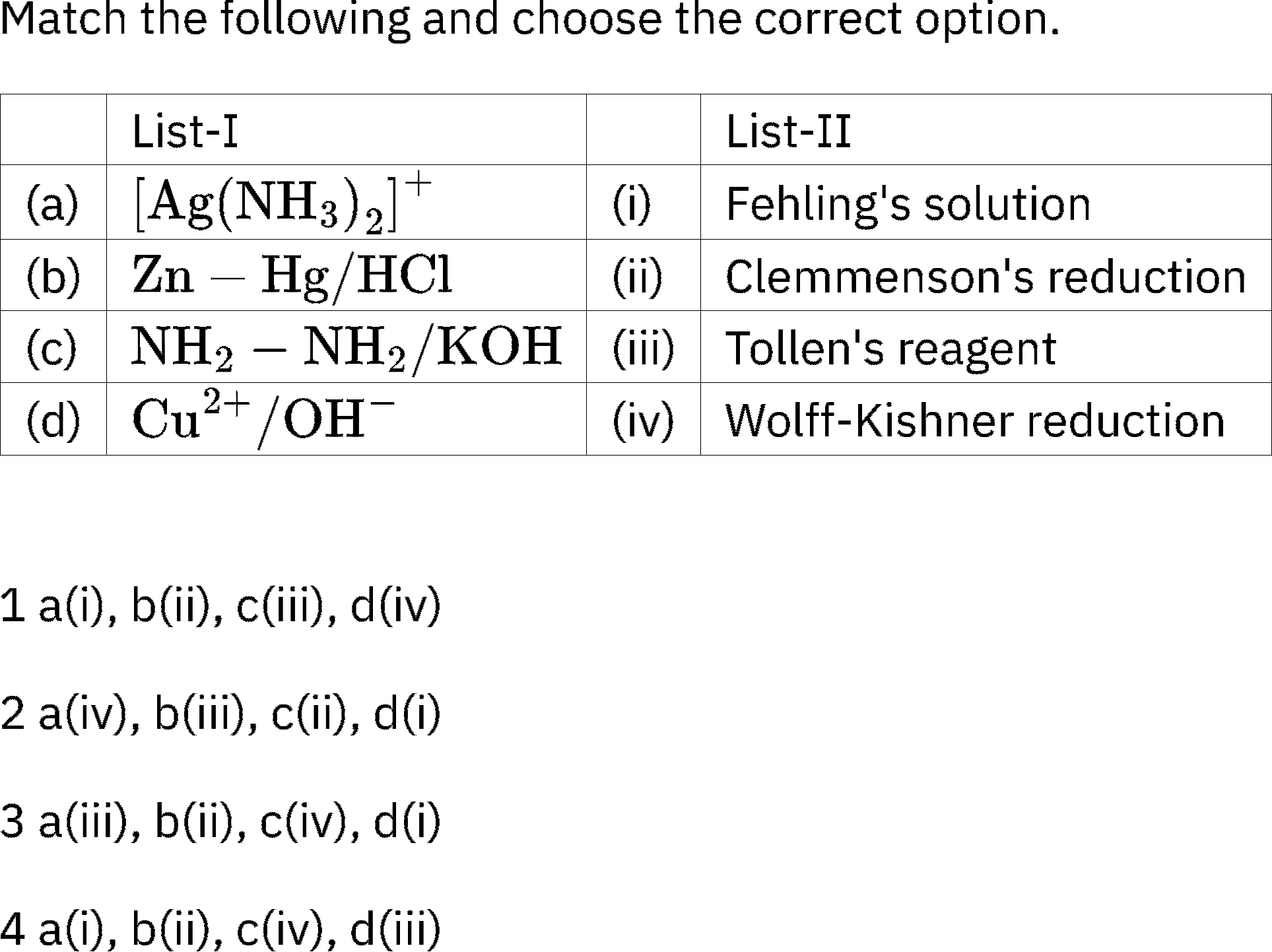
Question 1. Match List-I with List-II.
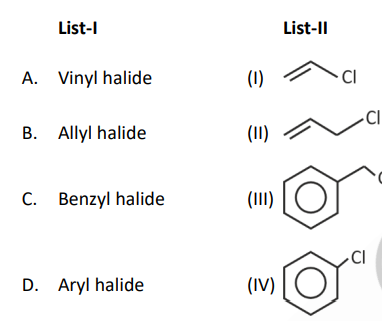
Select the correct option.
(1) A(II), B(I), C(III), D(IV)
(2) A(I), B(II), C(III), D(IV)
(3) A(I), B(II), C(IV), D(III)
(4) A(II), B(I), C(IV), D(III)
Answer (2)
Question 2. The correct order of stability of following diazonium ions is
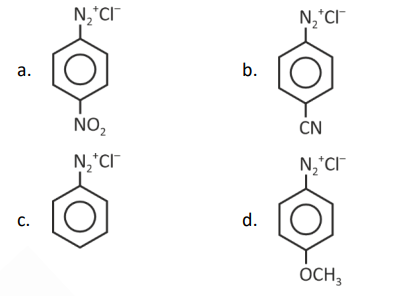
(1) a < b < c < d
(2) a < b < d < c
(3) c < d < b < a
(4) d < c < b < a
Stronger the electron withdrawing group attached at para position of
Stability:
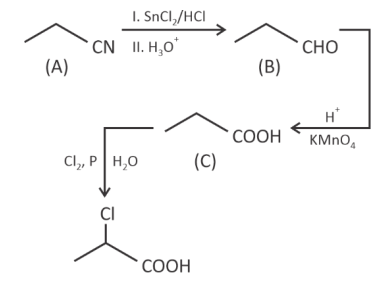
Question 3.
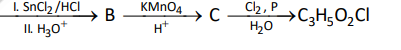
Final product has one chiral centre. Structure of A is
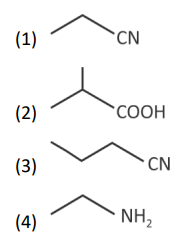
Answer (1)
Question 4. Which of following compound contains 3 unpaired electrons?
(1)
(2)
(3)
(4)
Answer (3)
Question 5. Consider the following molecules.
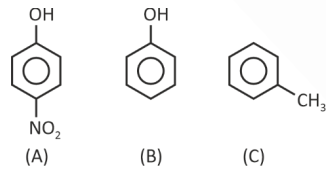
The correct order of dipole moment is
(1) A > B > C
(2) A > C > B
(3) B > A > C
(4) C > A > B
Answer (1)
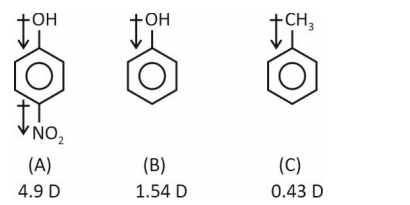
Dipole moment A > B > C
The JEE Mains 2026 Jan 22 evening shift was slightly tougher than the morning shift with Physics being the toughest of all three. Maths was easy and chemistry was moderate and overall the paper was moderate. You can view the in depth information by download the JEE Mains 2026 January 22 Shift 2 Question Paper analysis.
| Subject | Difficulty Level |
|---|---|
| Physics | Easy to Moderate |
| Chemistry | Moderate |
| Mathematics | Difficult |
| Overall | Moderate |
Some questions from Jan 22 Shift 2 listed below:Physics
Question 1:The correct order of electron gain enthalpy (magnitude only) for group 16 elements is
1 Te > Se > S > O
2 S > Se > Te > 0
3 O > S > Se > Te
4 S > O > Se > Te
Question 2: 100 g 98 % by weight H 2 SO 4 is mixed with 100 g 49 % by weight H 2 SO 4 . Mole fraction of H 2 SO 4 solution is
1 0.9
2 0.1
3 0.67
4 0.33
Question 3: 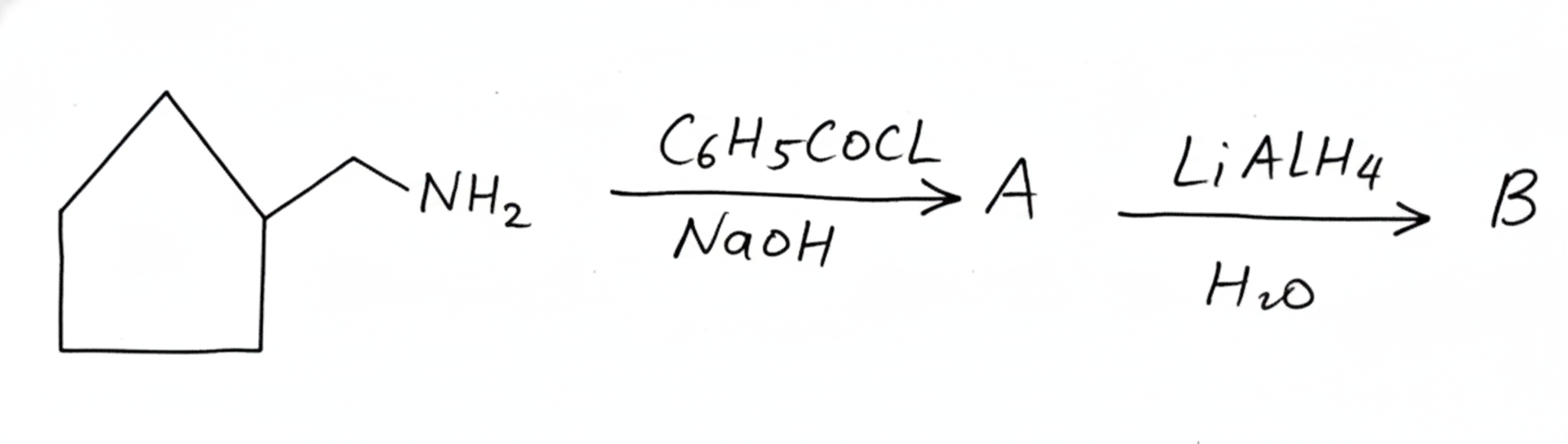
Question 4:
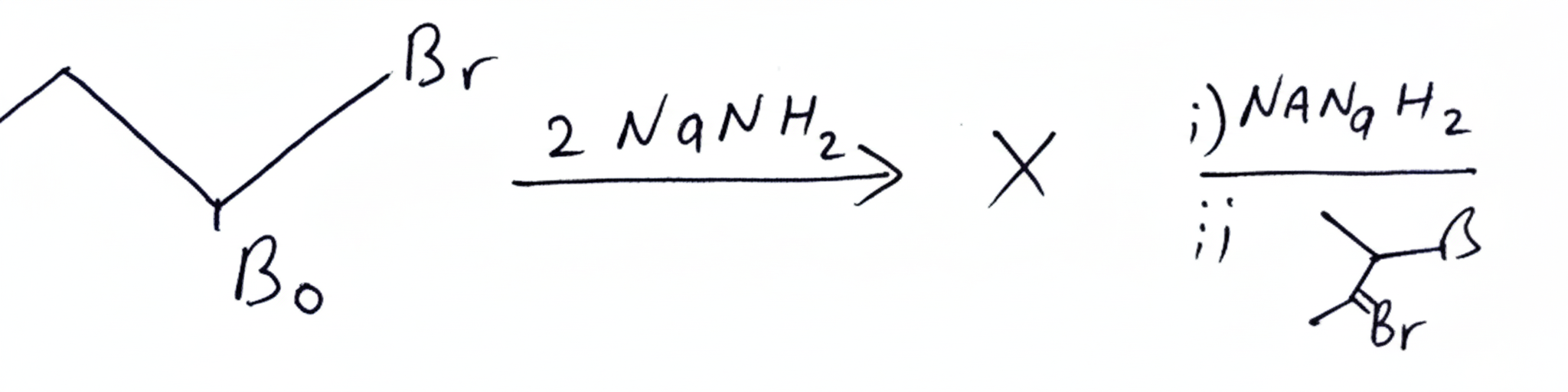
(1) isopropyl but-1-yne
(2) 2-Methyl hex-2-yne
(3) 5-Methyl Hex-2-yne
(4) 2-Methyl hex - 3-yne
Question 5: Correct order of ionisation enthalpy is
1 F > Cl > Cl − > F −
2 F − > Cl − > F > Cl
3 Cl > F > Cl − > F −
4 F > Cl − > F − > Cl −
JEE Main 2026 – January 22 Morning Shift was moderate to tough, with Physics being the most challenging, Mathematics lengthy and calculation-intensive, and Chemistry comparatively easier and more scoring. You can download the JEE Mains 2026 January 22 Question paper Shift 1also.
| Subject | Difficulty Level | Key Observations |
|---|---|---|
| Physics | Moderate to Difficult | Conceptual and time-consuming; tougher than Maths and Chemistry |
| Mathematics | Moderate | Lengthy calculations; required strong time management |
| Chemistry | Easy to Moderate | More scoring; Organic-heavy with direct questions |
Questions are provided below:Careers360 has provided you with memory-based questions below from the JEE Main 2026 January 22 Question Paper Shift 1. Along with analysis, we will be providing you with data from all subjects - Physics, Chemistry, and Maths.
Question 1: Consider the given central metal ions of low spin complex and choose the correct increasing order of unpaired electrons
(1)
(2)
(3)
(4)
Question 2:
Question 3: Which of the following is the correct order of the reactivity of given nucleophiles when treated with
Question 4: For the reaction given below at
Find
Given
(1) 0.43
(2) 0.23
(3) 0.31
(4) 0.53
Question 5:
Statement-I : Sucrose is dextrorotary and upon hydrolysis it becomes laevorotatory.
Statement-II : Sucrose on hydrolysis gives glucose and fructose such that the laevorotation of glucose is more than dextrorotation of fructose.
1 Both Statement-I and Statement-II are correct
2 Both Statement-I and Statement-II are incorrect
3 Statement-I is correct, Statement-II is incorrect
4 Statement-II is correct, Statement-I is incorrect
You can get the details by downloading JEE Main 2026 January 21 Question Paper Shift 2 with Solutions . The JEE Main 2026 January 21 Evening Shift paper was easy to moderate. The level of difficulty matched that of the January 2025 sessions. Among all the three subjects, Chemistry was the easiest, followed by Mathematics. Physics was the most scoring. Find more details below:
Subject | Overall Difficulty | Nature of Questions | Key Observations |
|---|---|---|---|
Physics | Easy to Moderate | Mostly formula-based, direct | Easier than morning shift and previous year; doable with proper revision |
Easy | Conceptual and statement-based | Easiest among all three subjects; revision-focused questions | |
Moderate to Hard | Lengthy and calculative | More time-consuming; required strong accuracy and time management |
Some memory based questions are given belQuestion 1: 1 g of an organic compound produce 1.49 of
Question 2: Given below are two statements
Statement-I : The correct order for radius is
Statement-II : Atomic size always, depends on electronegativity.
In the light of the above statements, choose the correct option.
1 Statement-I and II are correct
2 Both Statement-I and II are incorrect
3 Statement-I is correct but Statement-II incorrect
4 Statement-I is incorrect but Statement-II correct
Question 3: What will be significant figure of summation of
1) 3
2) 4
3) 5
4) 6
Question 4: When
Question 5: The rms speed of oxygen molecules at
(A) -100
(B) -253
(C) -20
(D) -235ow:
This shift was slightly tougher in terms of difficulty than last year. Some questions will be given below soon. You can check the JEE Mains Jan 21 Shift 1 Question Paper and the analysis table below:
| Parameter | Shift 1 |
|---|---|
| Overall Difficulty Level | Moderate to Tough |
| Physics Difficulty | Moderate |
| Chemistry Difficulty | Tough |
| Mathematics Difficulty | Moderate (easier than last year) |
| Numerical-Based Questions | Moderate number (mainly in Physics & Maths) |
| Conceptual Questions | High (especially in Chemistry) |
Some memory based questions are given belQuestion 1:
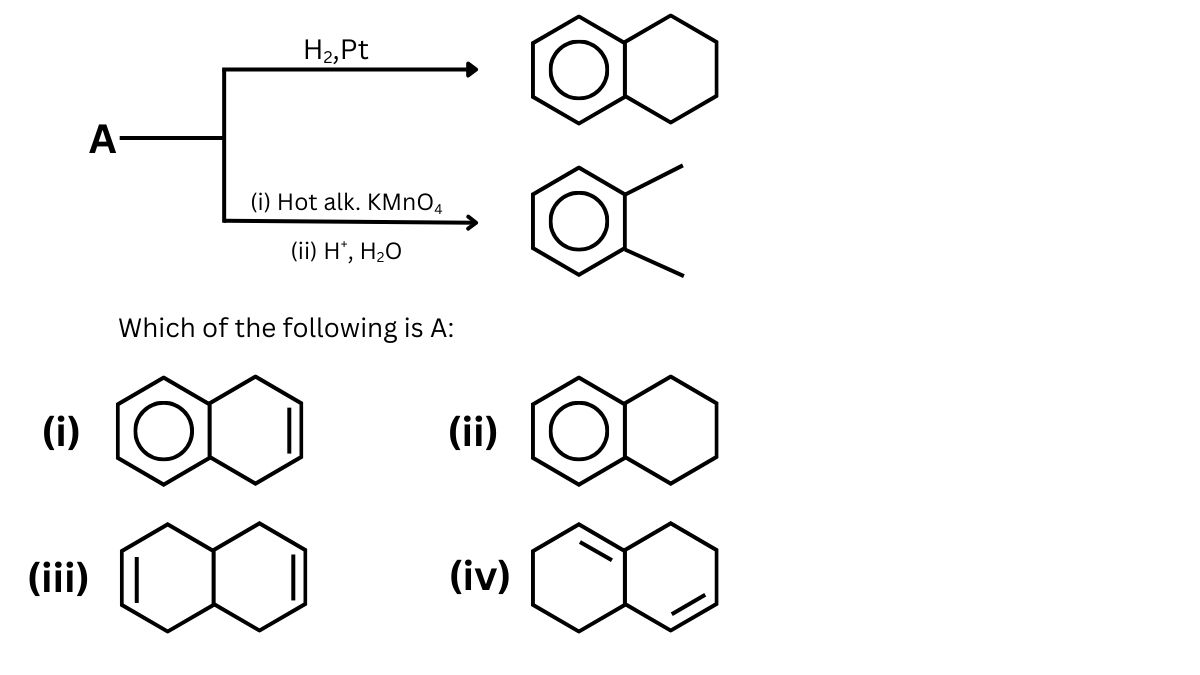
Solution: Option (1) is correct
Question 2: In Sulphur estimation, 0.7 g of an organic compound gives 1 g of BaSO4 by Carius method. What is the % of ‘S’ in compound?
(1) 19.61
(2) 23.85
(3) 27.93
(4) 14.57
Answer: Correct answer is option (1)
Question 3: Which of the following is the correct order with respect to the property indicated?
(1)
(2)
(3)
(4) None of these
Answer: Correct answer is option (2)
Question 4: In 'S' estimation 0.7 g of an organic compound gives 1 g BaSO4. in Carius method. What is the % of ' S ' in compound?
1) 19.61
2) 23.85
3) 27.93
4) 14.57
Question 5: Consider the following reaction
We have 14 g Ca reacts with excess of HCl . Choose the incorrect option.
(1) Mass of
(2) Mole of
(3) Volume of
(4) Mass of
Answer: Correct answer is option (4)
Question 6: Given below are two statements.
Statement 1 : Arginine and Tryptophan are essential amino acids.
Statement 1 : Glycine does not have any chiral carbon.
In the light of the above statements, which is the correct option.
Both statement-I and statement-II are correct
2 Both statement-I and statement-II are incorrect
3 Statement-I is correct and statement-II is incorrect
4 Statement-I is incorrect and statement-II is correctow:
The recent previous year question paper, i.e., JEE Mains 2025 question paper, must be solved by students before appearing for the JEE Mains paper 2026. It is an important study material for students which will help them understand how their preparation is coming along. You can view the JEE Mains 2025 question paper below:
In this section we will see the JEE Mains previous years question paper. This is for you to familiarize yourself to the type of questions to expect in your exam. There have been changes in the syllabus so you must only solve questions that are included in the JEE Mains 2026 syllabus. You can download the JEE Mains 2026 paper from the link below:
If you are studying subject wise and want to download the JEE 2026 Mains model question paper with detailed answers per subject, we have given it below:
You can also practice the JEE Mains 2026 sample paper:
Let us understand the JEE Mains exam pattern and also go through JEE Mains 2026 model questions:
Note: Incorrect answer will have negative marking of 1 from MCQs but there is no negative marking in case of incorrect answers in numerical questions.
Subject | Section | Question Type | No. of Questions | Marks |
Mathematics | Section A | MCQs | 20 | 80 |
Section B | Numerical Value | 5 | 20 | |
Physics | Section A | MCQs | 20 | 80 |
Section B | Numerical Value | 5 | 20 | |
Chemistry | Section A | MCQs | 20 | 80 |
Section B | Numerical Value | 5 | 20 | |
Total | 75 | 300 Marks |
Frequently Asked Questions (FAQs)
Yes, the JEE Main 2026 exam is going to be prepared by the NTA based on the same syllabus, unless changed, using the textbooks of NCERT.
You will get a total of 75 questions (25 each in the three subjects).
It will be carried out in two sessions- probably in January and April next year, 2026.
On this page, you can get PDF files of past years question papers with detailed solutions of last 10 years paper to refer easily. But for 2026, the JEE Main 2026 question paper with answers will be officially released by NTA a few days after the exam.
On Question asked by student community
decent chances actually as home state quota seats are 50%. allotments will depend on the JEE rank and not percentile though. in 2025, for female supernumerary it closed at 9286 rank while for open gen it closed at 5573.
So, would advise to use this tool to check the probable
yes you will. Ususally the return is within 7 days i it has failed at the gateway level which it seems to be. Please wait. You will get the money back
Slim chances as in 2025 the closing rank was 118 for SPA Delhi for B.Arch. You will need to wait for the rank list to come in April before getting a better picture. Please check https://engineering.careers360.com/jee-main-college-predictor for the predictions.
Check out https://engineering.careers360.com/jee-main-rank-predictor to know the probable rank
Hey Abhinav!
You can start your JEE Preparation with ICSE. You can check How to Prepare for JEE Main 2026?- Study Plan and start you preparation.
An 87.12% in JEE is good. To get admission to VIT, you'll need to take the VITEEE. You can apply for VITEEE if you meet the eligibility criteria for class 12. Your JEE score is not required for this process. Admission to VIT will be based on your VITEEE score.
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
70th University Ranked by NIRF | 80th Engineering Rank by NIRF | Accredited by NBA and NAAC A+
Last Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
Highest CTC 44.14 LPA | UGC Approved | 1600+ Recruiters | 100% Placement
NAAC A++ Grade | Recognized as Category-1 Deemed to be University by UGC | 41,000 + Alumni Imprints Globally