Amity University-Noida B.Tech Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
The Joint Entrance Examination (JEE) Main is conducted by the National Testing Agency (NTA), and every year, lakhs of students appear for the exam. It is the entrance test – or the preliminary selection round – for the top engineering colleges in the country including the Indian Institutes of Technology (IIT), National Institutes of Technology (NIT), Indian Institutes of Information Technology (IIIT) and many more government institutions.

Clearly, JEE Main is critical. However, JEE Main 2021 had questions which were conceptually or factually ambiguous and some of them were simply incorrect, going by the JEE Main answer key. Last year, the exam was conducted in four phases – February, March, July and August – across 26 Shifts. The result was declared in September.
Here, Careers360 looks into the questions asked in the July and August shifts, and highlights some of the errors in the JEE Main previous year question paper’s Chemistry section. The necessary changes required for these questions were absent from the NTA JEE Main answer keys, which could have affected the scorecards of many candidates in the JEE Main result.
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 26th March | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
These questions, along with their respective problems, are listed out below:
Question ID : 86435117255
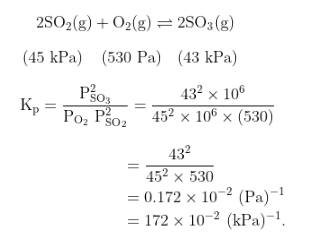
JEE Main Answer Key: 172
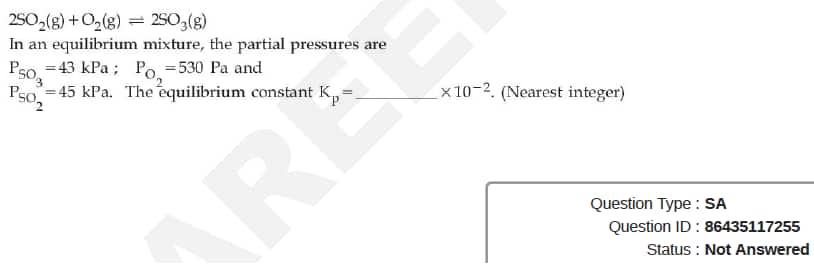
The issue:
The unit in which Kp is to be reported has not been specified in the question.
So, a student solving for the unit as (Pa)-1 will get an answer of 0.172 which would be rounded off to 0 while the given answer will be in units of (kPa)-1
____________________________________________________________________________
Question ID : 86435117528
JEE Main Answer Key: 1

The issue:
There is a conceptual error in the question. A negatively-charged sol cannot be precipitated by a negatively-charged ion.
Also, the coagulating value is defined as the minimum concentration of an electrolyte in millimoles per litre required to cause precipitation of a sol in two hours.
So, there arises another problem: How do you extrapolate data for two hours from the one-hour data?
____________________________________________________________________________
Question ID : 86435117785
JEE Main Answer Key: 86435159952 (Option 2)
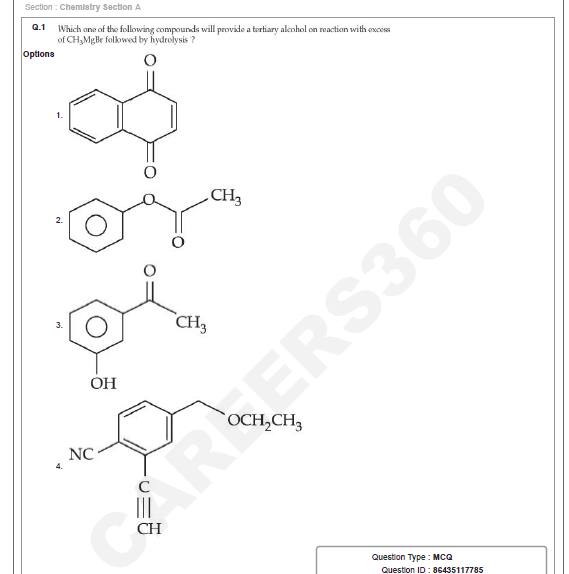
Question and Option IDs
The issue:
Multiple options given in the question (Options 1,2 and 3) are capable of forming tertiary alcohol on reaction with excess of Grignard reagent (CH3MgBr) followed by hydrolysis.
Option 1: Alpha- Beta unsaturated carbonyl compounds show direct addition to the carbonyl group with Grignard reagent. Hence, tertiary alcohols will be obtained from the carbonyl groups
Option 3: Grignard reagent can add to a ketone, forming a tertiary alcohol.
____________________________________________________________________________
Question ID : 86435118234
JEE Main Answer Key: 86435161299 (Option 1)
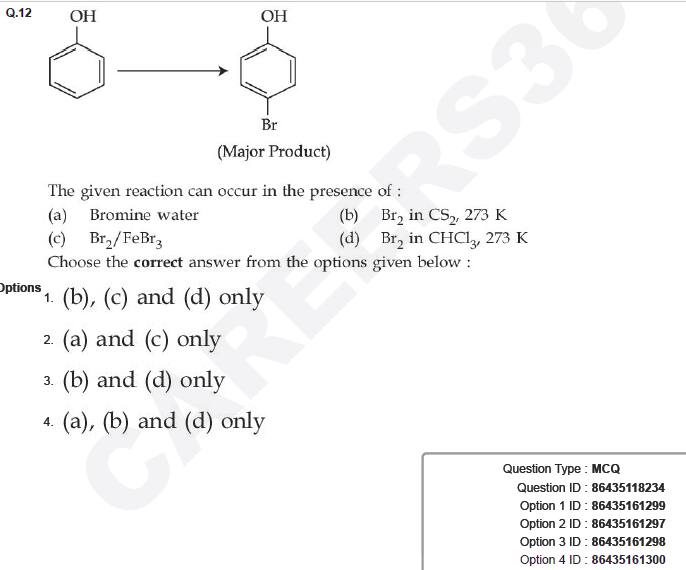
The issue:
Lewis acids are not used with Phenol because Phenol itself forms a complex with the Lewis acid, due to which the ring becomes deactivated towards Electrophilic Substitution.
In fact, this reaction of Phenol with a Lewis acid FeCl3 is actually used to detect the presence of Phenol in solution. Phenol forms a complex with neutral FeCl3 and produces a violet colour.
Correct answer should be 86435161298 [Option (3)]
____________________________________________________________________________
Question ID : 86435118225
JEE Main Answer Key: 86435161264 (Option 3)

The issue:
Be is not an alkaline earth metal as its oxide and hydroxide are Amphoteric in nature.
Here is an excerpt from NCERT textbook’s chapter on the s Block elements confirming it.

Hence, Statement I is also correct in the given question.
Correct answer should be 86435161261 [Option (2)]
____________________________________________________________________________
Question ID : 86435118870
JEE Main Answer Key: 5
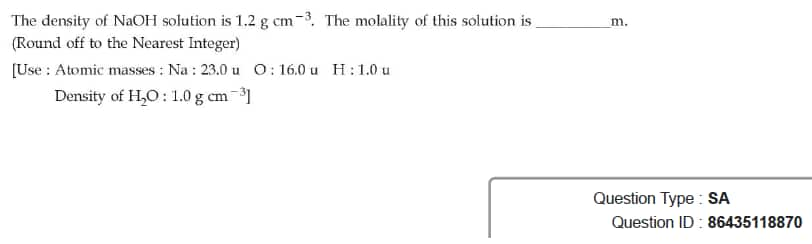
The issue:
Data given is insufficient to solve the problem.
Molality (m) can be calculated from the density of solution only when the Molarity (M) is also given in the question.
In the question, Molarity has not been provided and thus the question cannot be solved.
____________________________________________________________________________
Question ID : 86435119236
JEE Main Answer Key: 300
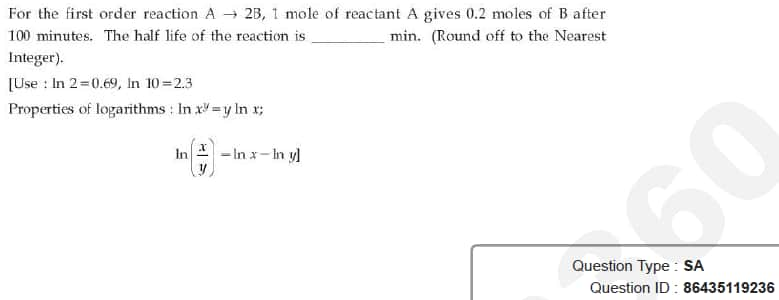
The issue :
The answer in the JEE Main final answer key was wrong. The answer should have been 600.

____________________________________________________________________________
Question ID : 86435120226
JEE Main Answer Key: 50
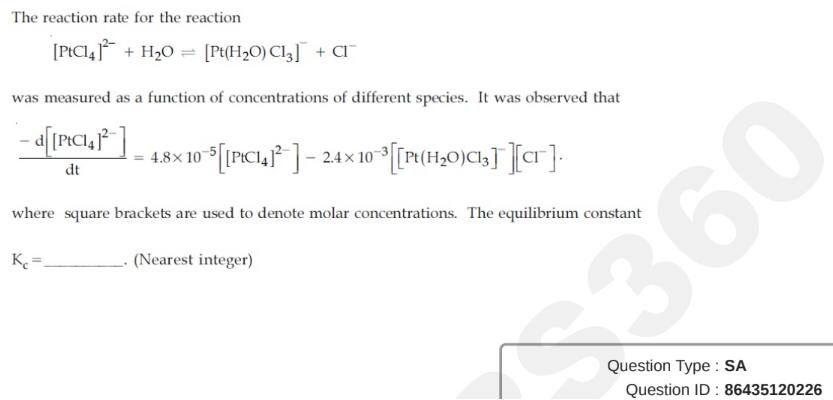
The issue :
The answer given in the answer key is wrong. The answer should be 0.
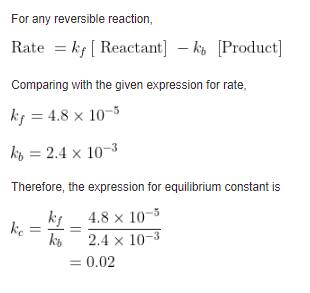
The answer is 0.02 which would be rounded off to 0.
For the answer to be 50, the value of (Kc)-1 had to be asked.
____________________________________________________________________________
Question ID : 86435120586
JEE Main Answer Key: 4
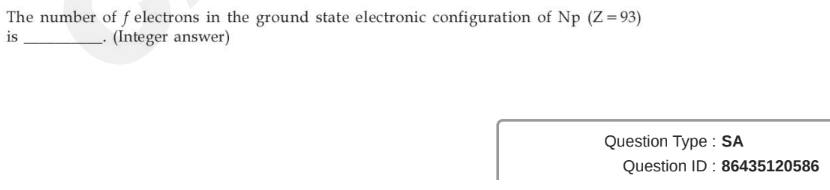
The issue :
The answer given is 4 because the inner 4f electrons have not been counted.
The question does not say that we need to consider only the 5f electrons
____________________________________________________________________________
Question ID : 86435121290
JEE Main Answer Key: 86435170463 (Option 4)
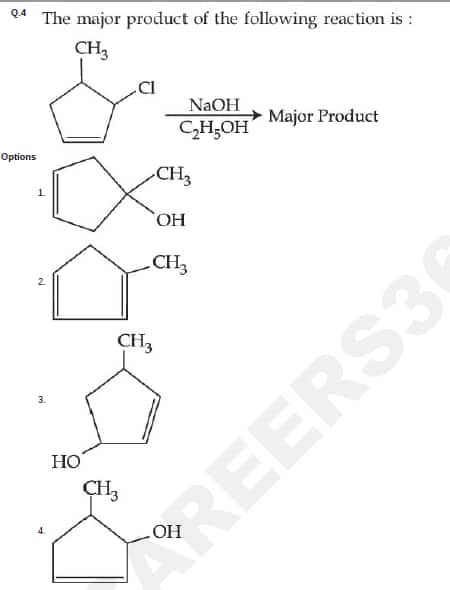
The issue :
Alcoholic NaOH causes dehydrohalogenation (elimination) and not substitution.
The answer should be 86435170462 (Option 2)
____________________________________________________________________________
Question ID : 86435121287
JEE Main Answer Key: 86435170452 (Option 2)
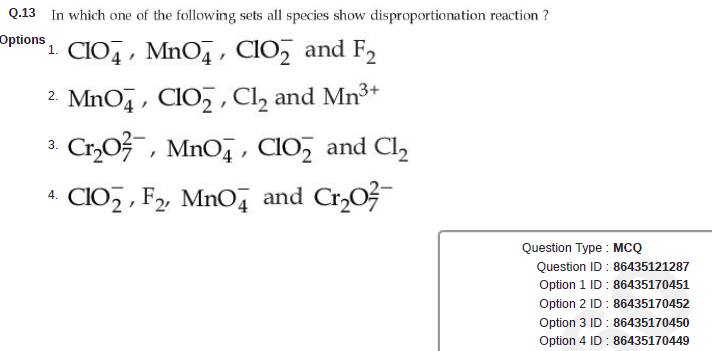
The issue :
None of the options is correct.
All the given options contain permanganate ion (MnO4-) which contains Mn in its highest oxidation state (+7) and hence, it cannot show disproportionation reaction
Question should have been awarded as bonus
____________________________________________________________________________
Question ID : 86435121562
JEE Main Answer Key: 86435171281 (Option 4)
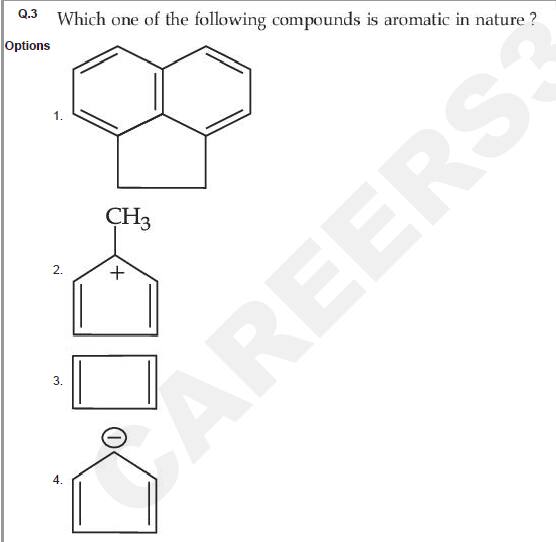
The issue :
Options 1 and 4 can both be correct but only Option 4 has been given as the correct answer.
Acenaphthene (compound in Option 1) is also Aromatic in nature.
____________________________________________________________________________
Apart from the above-mentioned questions, there were also some in the integer section that had very lengthy mathematical calculations and whose answers had to be rounded off to the nearest integers.
These questions are usually solved by approximations and it is very difficult for students to arrive at the correct integer answer. Previously, these questions had a correct range for the answer, which gave students some leeway to make appropriate approximations in calculations.
Now, with negative marking in the numerical response questions, this becomes an even bigger problem. Considering the high-stakes nature of the JEE Mains, even a few such errors can have significant ramifications for applicants and be deeply demoralising.
On Question asked by student community
Usha Mittal of Technology has no AI quota officially. please contact the college for any mangement quota seats.
decent chances actually as home state quota seats are 50%. allotments will depend on the JEE rank and not percentile though. in 2025, for female supernumerary it closed at 9286 rank while for open gen it closed at 5573.
So, would advise to use this tool to check the probable
yes you will. Ususally the return is within 7 days i it has failed at the gateway level which it seems to be. Please wait. You will get the money back
Slim chances as in 2025 the closing rank was 118 for SPA Delhi for B.Arch. You will need to wait for the rank list to come in April before getting a better picture. Please check https://engineering.careers360.com/jee-main-college-predictor for the predictions.
Check out https://engineering.careers360.com/jee-main-rank-predictor to know the probable rank
Hi Smita Sharma,
With 47 percentile in JEE Mains 2026, you might get rank around 7,00,000 plus which is very high. Check the link below for the Best engineering colleges available for you based on yours percentile.
Link 1: https://engineering.careers360.com/colleges/list-of-engineering-colleges-in-pune-accepting-jee-main
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Recognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
70th University Ranked by NIRF | 80th Engineering Rank by NIRF | Accredited by NBA and NAAC A+
Last Date to Apply: 26th March | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
Highest CTC 44.14 LPA | UGC Approved | 1600+ Recruiters | 100% Placement
NAAC A++ Grade | Recognized as Category-1 Deemed to be University by UGC | 41,000 + Alumni Imprints Globally