You passed 12th in 2024 as a PCB student and now plan to appear again in 2026 with PCM through NIOS.
For JEE Main 2026, you will be eligible because students can appear in the year they pass 12 and up to two more years.
Since you are giving
JEE Advanced Chemistry 2025 Analysis - Top Skipped, Wrongly Attempted and Correctly Solved Questions
JEE Advanced Chemistry 2025 Analysis: Are you preparing for JEE Advanced? If the answer is yes then it is important to clear concepts and solve questions after that understanding of past year trends can give you an advantage over others. Along with theoretical knowledge students must focus on problem solving methods, and time management for clearing JEE Advanced. Identification of types of questions that are frequently skipped, wrongly attempted, or solved correctly gives a clear idea of how students performed and shows which parts of the paper were the toughest in JEE Advanced 2025 Chemistry. The registration for JEE Main 2026 has already started, and students can register from 31 October 2025 to 27 November 2025. Session 1 of the exam will be conducted from 21 to 30 January 2026.
This Story also Contains
- JEE Advanced 2025 Chemistry Analysis - Paper 1 and Paper 2 Question-Wise Candidate’s Performance
- Most Skipped Chemistry Questions (High Non-Attempt %)
- Most Wrongly Attempted Chemistry Questions (High Wrong Response %)
- Most Correctly Solved Chemistry Questions (Higher Accuracy)
- Distribution of Total Marks in Aggregate (all, qualified, and allotted candidates)
- JEE Advanced 2025 Chemistry: Most Difficult Questions

JEE Advanced 2025 Chemistry Analysis - Paper 1 and Paper 2 Question-Wise Candidate’s Performance
Chemistry of JEE Advanced 2025 in both Paper 1 and Paper 2 is a perfect blend of simple and tricky questions. Given below a closer look at candidate wise performance on which types of questions were solved accurately, skipped frequently, or answered incorrectly from Chemistry.
Paper 1/2 | Question (2025) | Not Attempted | % Not Attempted | Full Marks | % Full Marks | Partial Marks | % Partial Marks | Wrong Response |
1 | Q.1 | 69494 | 38.52 | 61228 | 33.94 | 49700 | ||
1 | Q2 | 49702 | 27.55 | 85090 | 47.16 | 45630 | ||
1 | Q3 | 42763 | 23.7 | 81216 | 45.01 | 56443 | ||
1 | Q4 | 40324 | 22.35 | 54822 | 30.39 | 85276 | ||
1 | Q5 | 26693 | 14.79 | 29921 | 16.58 | 26305 | 14.58 | 97503 |
1 | Q6 | 60319 | 33.43 | 50456 | 27.97 | 30410 | 16.85 | 39237 |
1 | Q7 | 59779 | 33.13 | 70100 | 38.85 | 0 | 0 | 50543 |
1 | Q8 | 31189 | 17.29 | 46128 | 25.57 | 103105 | ||
1 | Q9 | 39526 | 21.91 | 2295 | 1.27 | 138601 | ||
1 | Q10 | 68500 | 37.97 | 4623 | 2.56 | 107299 | ||
1 | Q11 | 39742 | 22.03 | 7372 | 4.09 | 133308 | ||
1 | Q12 | 61356 | 34.01 | 21739 | 12.05 | 97327 | ||
1 | Q13 | 73051 | 40.49 | 871 | 0.48 | 106500 | ||
1 | Q14 | 64795 | 35.91 | 92876 | 51.48 | 22751 | ||
1 | Q15 | 21895 | 12.14 | 99348 | 55.06 | 59179 | ||
1 | Q16 | 63560 | 35.23 | 90312 | 50.06 | 26550 | ||
2 | Q1 | 50927 | 28.23 | 54561 | 30.24 | 74934 | ||
2 | Q2 | 58469 | 32.41 | 56427 | 31.28 | 65526 | ||
2 | Q3 | 62405 | 34.59 | 35693 | 19.78 | 82324 | ||
2 | Q4 | 42895 | 23.77 | 50535 | 28.01 | 86992 | ||
2 | Q5 | 76736 | 42.53 | 5729 | 3.18 | 33946 | 18.81 | 64011 |
2 | Q6 | 14785 | 8.19 | 103996 | 57.64 | 30163 | 16.72 | 31478 |
2 | Q7 | 79874 | 44.27 | 28321 | 15.7 | 19615 | 10.87 | 52612 |
2 | Q8 | 60685 | 33.64 | 23533 | 13.04 | 27866 | 15.44 | 68338 |
2 | Q9 | 51822 | 28.72 | 18287 | 10.14 | 110313 | ||
2 | Q10 | 53775 | 29.81 | 12980 | 7.19 | 113667 | ||
2 | Q11 | 64244 | 35.61 | 5663 | 3.14 | 110515 | ||
2 | Q12 | 51706 | 28.66 | 20645 | 11.44 | 108071 | ||
2 | Q13 | 44997 | 24.94 | 14813 | 8.21 | 120612 | ||
2 | Q14 | 36717 | 20.35 | 3915 | 2.17 | 139790 | ||
2 | Q15 | 8324 | 4.61 | 42441 | 23.52 | 129657 | ||
2 | Q16 | 55074 | 30.53 | 32631 | 18.09 | 92717 |
Most Skipped Chemistry Questions (High Non-Attempt %)
Some JEE Advanced Chemistry questions were left unanswered by a large number of candidates, reflecting their difficulty level or time consuming nature. Analysing these high non-attempt percentage questions helps identify the topics that are difficult to understand.
Paper 1
Question Number | % Not Attempted | Chapter Name | Concept Name |
Q13 | 40.49 | Aldehydes, Ketones, And Carboxylic Acid | Williamson's ether synthesis |
Q10 | 37.97 | States Of Matter | Vander Waals equation |
Q14 | 35.91 | P- Block Elements | Salt analysis |
Q16 | 35.23 | Amines | Tests for functional groups |
Q12 | 34.01 | Organic chemistry some basic principles and techniques | Nitrogen estimation and carbylamine test |
Paper 2
Question Number | % Not Attempted | Chapter Name | Concept Name |
Q7 | 44.27 | Amines | Gabriel phthalamide synthesis |
Q5 | 42.53 | States Of Matter | intermolecular forces |
Q11 | 35.61 | Surface Chemistry | Freundlich adsorption isotherm |
Q3 | 34.59 | Aldehydes, Ketones And Carboxylic Acid | Named reactions Aldol condensation, oxymercuration, ozonolysis |
Q8 | 33.64 | Aldehydes, ketones and carboxylic acids | Reduction, oxidation, dehydration reactions |
JEE Advanced Chemistry Paper 2 Question 7
Question 7 of Chemistry JEE Advanced is one of the key problems that tested both conceptual clarity and application skills
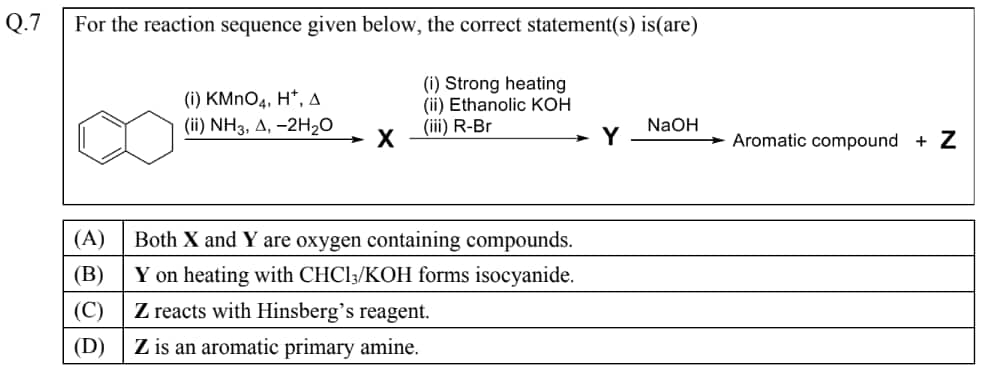
Solution:
Starting naphthalene → KMnO 4 / H + , Δ → phthalic acid → NH 3 , Δ ( − 2 H 2 O ) → X = phthalimide.
Phthalimide ( X ) → KOH / EtOH → N -alkylation with R − Br → Y = N -alkylphthalimide (Gabriel synthesis).
NaOH hydrolysis of Y → phthalate (aromatic) + Z = RNH 2 (primary amine).
So,
(A) X and Y both contain oxygen → True.
(B) Y is not a primary amine → no carbylamine test → False.
(C) Z is a primary amine, gives Hinsberg reaction → True.
(D) Z is aliphatic (Gabriel with R − Br = alkyl); aryl halides don't undergo SN 2 → False.
Hence, the correct answers are option 1,3.
Why most students skip this question?
1. First step to solve this question is Multi step Conversion where the reaction required linking oxidation → phthalimide formation → Gabriel synthesis → hydrolysis.
2. The key intermediate here is phthalimide, which is not a very common compound in everyday practice questions. Since many students had not memorized that naphthalene oxidation → phthalic acid → phthalimide, they struggled to identify “X” confidently.
3. In this question Option (B) tempted students with the carbylamine test, but the intermediate Y is not a primary amine. While option (D) suggested Z is an aromatic amine, but Gabriel synthesis doesn’t yield aryl amines.
4. This questions was placed among organic reaction sequence questions, which are usually time-consuming. Many aspirants skipped it to avoid losing time on an unfamiliar, lengthy pathway, preferring quicker physical or inorganic chemistry questions.
Most Wrongly Attempted Chemistry Questions (High Wrong Response %)
Some Chemistry JEE Advanced 2025 questions have a high percentage of wrong responses, which shows common misconceptions and calculation errors among candidates. Analysing these questions helps students understand the tricky concepts.
Paper 1
Question Number | % Wrong Response | Chapter Name | Concept Name |
Q9 | 76.82 | Equilibrium | Weak acid dissociation |
Q11 | 73.89 | Thermodynamics | Expansion work of ideal gas |
Q10 | 59.47 | States Of Matter | Vander waals equation |
Q13 | 59.03 | Aldehydes, Ketones And Carboxylic Acid | Williamsons ether synthesis |
Q8 | 57.15 | Electrochemistry | Electrolysis and faradays law |
Paper 2
Question Number | % Wrong Response | Chapter Name | Concept Name |
Q14 | 77.48 | Electrochemistry | Relation between gibbs free energy and cell potential |
Q15 | 71.86 | Coordination Compounds | Crystal field theory |
Q13 | 66.85 | Solutions | Osmotic pressure |
Q11 | 61.25 | Surface Chemistry | Freundlich adsorption isotherm |
Q12 | 59.9 | Chemical Kinetics | Pseudo-first-order reaction |
JEE Advanced Chemistry Paper 2 Question 14
Question 14: An electrochemical cell is fueled by the combustion of butane at 1 bar and 298 K . Its cell potential is X F × 10 3 volts, where F is the Faraday constant. The value of X is .
Use: Standard Gibbs energies of formation at 298 K are: Δ f G CO 2 o = − 394 kJ mol − 1 ; Δ f G water o = − 237 kJ mol − 1 ; Δ f G butane o = − 18 kJ mol − 1
Solution:
Given
Combustion fuel cell at 298 K , 1 bar .
C 4 H 10 + 13 2 O 2 → 4 CO 2 + 5 H 2 O ( l )
Δ f G ∘ ( CO 2 ) = − 394 kJ mol − 1 , Δ f G ∘ ( H 2 O ( l ) ) = − 237 kJ mol − 1 , Δ f G ∘ ( C 4 H 10 ) = − 18 kJ mol − 1
1) Reaction Gibbs energy
Δ G rxn ∘ = [ 4 ( − 394 ) + 5 ( − 237 ) ] − ( − 18 ) + 13 2 ( 0 )
= ( − 1576 − 1185 ) − ( − 18 )
= − 2761 + 18
= − 2743 kJ mol − 1
2) Electrons transferred n
Average oxidation state of C in butane: 4 x + 10 ( + 1 ) = 0 ⇒ x = − 2.5 .
In CO 2 , C is +4 . Change per C = + 6.5 .
For 4 carbons: n = 4 × 6.5 = 26 electrons.
(Equivalently, for C a H b O c : n = 4 a + b − 2 c = 4 ⋅ 4 + 10 − 0 = 26 .)
3) Cell potential
E ∘ = − Δ G rxn ∘ n F = 2743 kJ mol − 1 26 F = 105.5 F × 10 3 V
so X = 105.5 .
(For reference, E ∘ ≈ 1.09 V using F = 96485 C mol − 1 .)
Hence, the correct answer is 105.5
Why many students got it wrong?
1. The question required combining Gibbs energy calculations with electrochemistry concepts. Many students are comfortable with each part separately, but finding and applying the link between Δ G ∘ of reaction → cell potential can be difficult.
2. Students often do mistakes in Balancing of reactions or Electron Count
3. Errors may arise in the step where Δ G ∘ for the overall reaction is calculated using standard Gibbs free energies of formation.
4. This question involves multiple calculation steps like reaction balancing → Δ G ∘ → electrons transferred → unit conversions, under time constraints students tend to skip detailed re-checks. Any small slip along the way compounds to an incorrect final value.
Most Correctly Solved Chemistry Questions (Higher Accuracy)
Some JEE Advanced 2025 Chemistry questions were answered correctly by the majority of candidates, showing clear understanding and strong command over fundamental concepts.
Paper 1
Question Number | % Full Marks | Chapter Name | Concept Name |
Q15 | 55.06 | Amines | Named reactions |
Q14 | 51.48 | P- Block Elements | Salt analysis |
Q16 | 50.06 | Amines | Tests for functional groups |
Q2 | 47.16 | Coordination Compounds | Spectrochemical series and electronic transition |
Q3 | 45.01 | P- Block Elements | Redox reactions of KMnO4 |
Paper 2
Question Number | % Full Marks | Chapter Name | Concept Name |
Q6 | 57.64 | P- Block Elements | oxyacids of phosphorus |
Q2 | 31.28 | P- Block Elements | Hydrolysis of interhalogens |
Q1 | 30.24 | Coordination compounds | Sodium nitroprusside test |
Q4 | 28.01 | Alcohols, Phenols And Ether | oxidative clevage |
Q15 | 23.52 | Coordination Compounds | Crystal field theory |
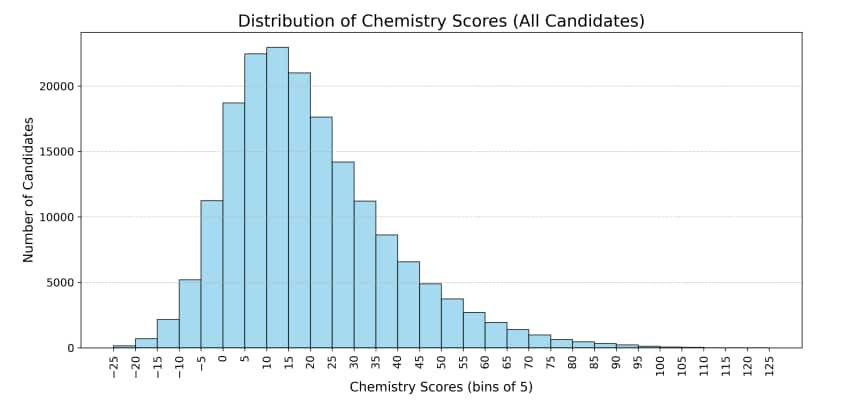
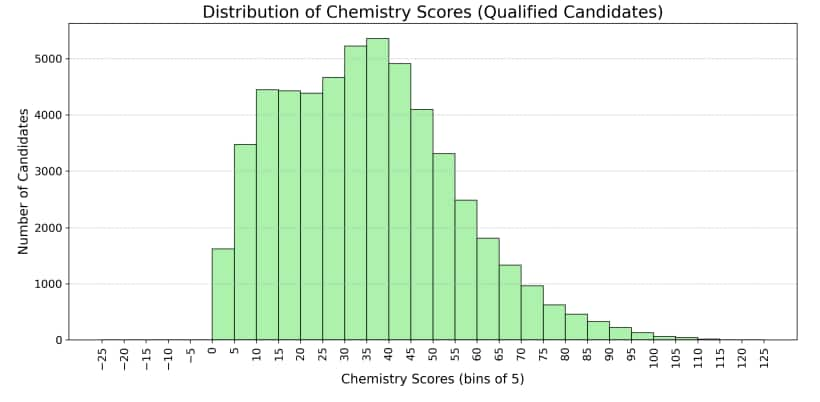
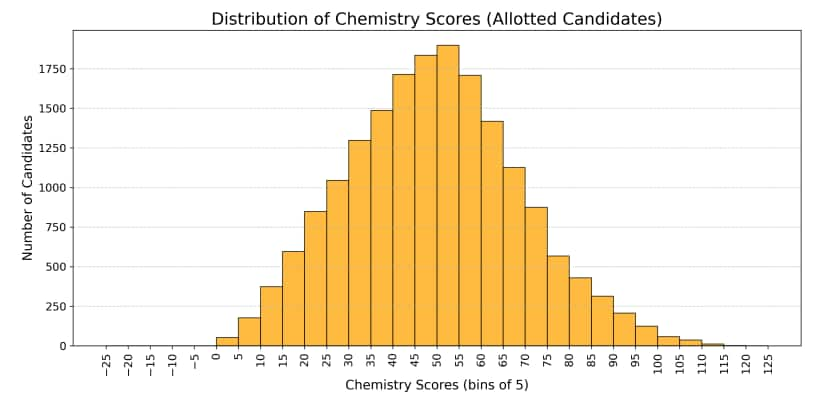
Distribution of Total Marks in Aggregate (all, qualified, and allotted candidates)
Below is the total marks distribution in Chemistry JEE Advanced 2025 that shows how candidates performed overall, including those who qualified and those who were allotted seats.
For All Candidates
For Qualified Candidates
For Seat Allotted Candidates
JEE Advanced 2025 Chemistry: Most Difficult Questions
Some Chemistry questions in JEE Advanced 2025 were difficult, testing understanding and problem solving skills of students. These questions had high non-attempt or wrong response rates, making them the toughest for candidates.
Paper 1
Q. No | % Not Attempted | % Full Marks | % Wrong Response | Chapter | Concept |
Q13 | 40.49 | 0.48 | 59.03 | Aldehydes, Ketones And Carboxylic Acid | Williamsons ether synthesis |
Q11 | 22.03 | 4.09 | 73.89 | Thermodynamics | Expansion work of ideal gas |
Q10 | 37.97 | 2.56 | 59.47 | States Of Matter | Vander waals equation |
Q9 | 21.91 | 1.27 | 76.82 | Equilibrium | Weak acid dissociation |
The most difficult questions in JEE Advanced chemistry Paper 1 (Q13, Q11, Q10, Q9) turned out to be challenging due to a mix of high wrong response rates and very low full marks percentages. Questions 9 and 11 have, on average, 75.35% wrong attempts. This shows that students found the questions easy, but they fell into the trap. As a result, they ended up making wrong attempts. And Question 13 is the least full-mark (0.48%) question.
Paper-2
Q. No | % Not Attempted | % Full Marks | % Wrong Response | Chapter | Concept |
Q1 | 28.23 | 30.24 | 41.53 | Coordination compounds | Sodium nitroprusside test |
Q2 | 32.41 | 31.28 | 36.32 | P- Block Elements | Hydrolysis of interhalogens |
Q8 | 33.64 | 13.04 | 37.88 | Aldehydes, ketones and carboxylic acids | Reduction, oxidation, dehydration reactions |
Q4 | 23.77 | 57.64 | 48.22 | Alcohols, Phenols And Ether | oxidative cleavage |
Frequently Asked Questions (FAQs)
The top skipped questions often include those that are conceptually challenging or require intricate calculations. In 2025, many candidates tended to skip questions related to coordination chemistry and complex reaction mechanisms. Such questions demand not only understanding but also the ability to apply this knowledge under time constraints.
Students can adopt several strategies to tackle complex topics. First, breaking down challenging concepts into smaller, manageable parts makes them easier to comprehend. Using visual aids, like charts and diagrams, can help in understanding intricate processes. Additionally, practicing with varied problems and scenarios reinforces knowledge.
To improve performance, candidates should focus on enhancing their conceptual understanding through targeted study. Practicing past years’ papers and taking mock tests can help identify weak areas. Additionally, forming study groups or seeking help from mentors can clarify complex concepts. It’s also vital for students to time themselves during practice to simulate exam conditions.
Candidates performed particularly well on questions that involved stoichiometric calculations and basic principle-based questions. These topics are generally more straightforward and align closely with the foundational concepts taught in many chemistry courses. Questions requiring direct applications of laws, like the mole concept or basic thermodynamics, were often answered correctly, suggesting that students had a strong grasp of these fundamental ideas.
Yes, certain topics showed a higher incidence of wrongly attempted questions. Organic chemistry, particularly reaction mechanisms and stereochemistry, was a common hurdle for many students. The complexity of these concepts can lead to confusion and mistakes, especially if a student misapplies a rule or doesn't fully grasp the underlying principles.
Popular Courses and Specializations
List of colleges accepting JEE Advanced
Browse Engineering Colleges by State
Questions related to JEE Advanced
On Question asked by student community
To get admission into IIT Bombay , a students belonging to economically weaker section (EWS) should score minimum 108-160+ marks in JEE Advanced examination.
Only the top 2.5 lakh students will be eligible for JEE Advanced. But the qualifying cutoff for JEE Advanced will be out once the JEE Main Session 2 results are out. At this rank, you might not be eligible for the JEE Advanced exam, so we suggest you go for
The JEE Advanced result will be released on June 1, 2026, at 10 AM. The examination will be conducted on May 17, 2026. The JEE Advanced results will be released on the official website at jeeadv.ac.in. To login, you need to enter your application number and date of birth.
You can download the JEE Advanced previous year questions with solutions from the Careers360 website. I am providing the link below.
https://engineering.careers360.com/articles/jee-advanced-question-papers
Hello
B.Tech/B.Arch Admissions OPEN
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Amrita University B.Tech 2026
ApplyRecognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
Vignan's Deemed to be University B.Tech Admissions 2026
Apply70th University Ranked by NIRF | 80th Engineering Rank by NIRF | Accredited by NBA and NAAC A+
UPES B.Tech Admissions 2026
ApplyLast Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
MIT World Peace University B.Tech Admissions 2026
ApplyHighest CTC 44.14 LPA | UGC Approved | 1600+ Recruiters | 100% Placement
Manav Rachna-B.Tech Admissions 2026
ApplyNAAC A++ Grade | Recognized as Category-1 Deemed to be University by UGC | 41,000 + Alumni Imprints Globally
