IUPAC Nomenclature of Alkanes - Practice Questions & MCQ
Quick Facts
-
6 Questions around this concept.
Solve by difficulty
The IUPAC name of  is
is
Concepts Covered - 1
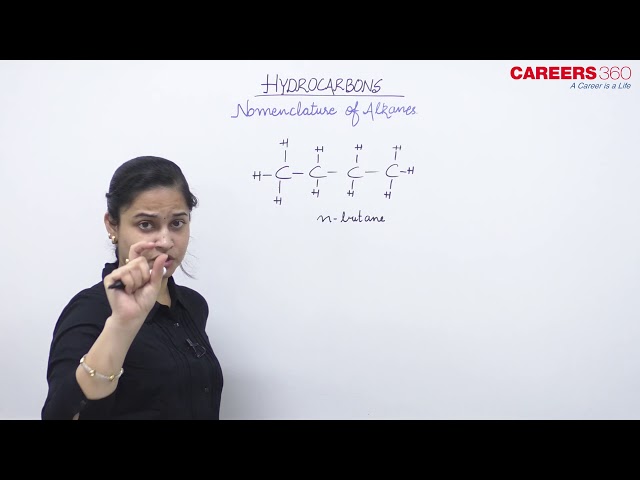
Nomenclature of straight-chain hydrocarbons
The names of such compounds are based on their chain structure, and end with suffix ‘-ane’ and carry a prefix indicating the number of carbon atoms present in the chain (except from CH4 to C4H10, where the prefixes are derived from trivial names). The IUPAC names of some straight-chain saturated hydrocarbons are given in the Table below. The alkanes in this Table differ from each other by merely the number of -CH2 groups in the chain. They are homologues of alkane series.

Nomenclature of branched-chain alkanes:
The rules for naming branched-chain alkanes are as follows:
-
First of all, the longest carbon chain in the molecule is identified. In the example given below, the longest chain has nine carbons and it is considered as the parent or root chain.

-
The carbon atoms of the parent chain are numbered to identify the parent alkane and to locate the positions of the carbon atoms at which branching takes place due to the substitution of alkyl group in place of hydrogen atoms. The numbering is done in such a way that the branched carbon atoms get the lowest possible numbers. Thus, the numbering in the above example should be from left to right (branching at carbon atoms 2 and 6).
-
The names of alkyl groups attached as a branch are then prefixed to the name of the parent alkane and position of the substituents is indicated by the appropriate numbers. If different alkyl groups are present, they are listed in alphabetical order. Thus, name for the compound shown above is: 6-ethyl-2-methylnonane.
-
If two or more identical substituent groups are present then the numbers are separated by commas. The names of identical substituents are not repeated, instead prefixes such as di (for 2), tri (for 3), tetra (for 4), penta (for 5), hexa (for 6) etc. are used. While writing the name of the substituents in alphabetical order, these prefixes, however, are not considered.
-
If the two substituents are found in equivalent positions, the lower number is given to the one coming first in the alphabetical listing. Thus, the following compound is 3-ethyl-6-methyloctane and not 6-ethyl-3-methyloctane.

Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"