Adsorption and Degree of Unsaturation - Practice Questions & MCQ
Quick Facts
-
10 Questions around this concept.
Solve by difficulty
The total number of primary, secondary and tertiary amines possible with the molecular formula C3H9N respectively
What is the general formula of aromatic compounds?
Compound with molecular formula C3H6O can show :
New: JEE Main 2026 Answer Key Out; Download Now
JEE Main 2026 Tools: Rank Predictor | College Predictor
JEE Main 2026: Session 2 Registration Link | Session 1 Official QP
Consider the substrate given below:

This is allowed to undergo hydrogenation reaction in presence of metals like Ni or Pt as catalyst The difference in the degree of unsaturation of the reactant and product is
Concepts Covered - 1
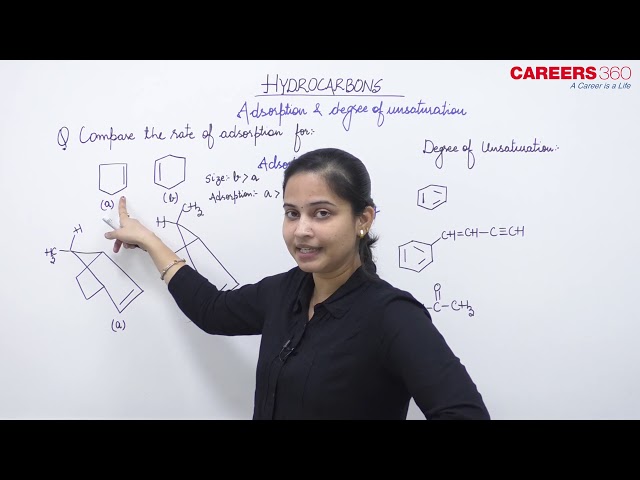
Adsorption
The phenomenon of the deposition of particles on the surface of the adsorbent is called adsorption.
For example, the rate of adsorption among the following molecules is higher for cyclopentene.


Cyclopentene Cyclohexene
Since the size of cyclopentene is smaller than cyclohexene, thus the rate of adsorption is higher for cyclopentene than cyclohexene.
Occlusion
The adsorption of hydrogen on the surface of the noble metals is known as occlusion. These noble metals can be Platinum or Palladium.
Degree of Unsaturation
It is the calculation to analyse the total number of double bonds and rings in the organic compound. It is also known as index of hydrogen deficiency or unsaturation index.
- The degree of unsaturation for simple compounds like benzene is given as:
Degree of unsaturation = Number of pi bonds + Number of rings
Thus, Degree of unsaturation = 3 + 1 = 4 (Since there are 3 double bonds in benzene ring and 1 ring is only present) - For complex compounds like C10H6N4, the degree of unsaturation is given as follows:
These complex compounds can be represented as CxHyNzOpXq. Now, the degree of unsaturation for complex compounds are given using the following formula:
Where, C is the number of Carbon atoms, H is the number of Hydrogen atoms, N is the number of Nitrogen atoms and X is the number of Halogen atoms. It is to be noted that the Oxygen atoms have no bearing on the degree of unsaturation
Now for the compound C10H6N4 we have, x = 10, y = 6 and z = 4
Thus, degree of unsaturation = (10 + 1) - (6 + 0 - 4)/2
Degree of unsaturation = 10
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"