Thermodynamic Property - Practice Questions & MCQ
Quick Facts
-
11 Questions around this concept.
Solve by difficulty
If there is no interaction of mass and energy between system and surroundings, then system is know as:
In a closed system, which of the following can transfer from system to surroundings -
Which of the following are intensive property:
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
Which of the following is an extensive property:
Among the quantities, density $(\rho)$, temperature $(T)$, enthalpy $(H)$, heat capacity $\left(C_\rho\right)$, volume $(V)$ and pressure $(P)$, a set of intensive variables are:
Concepts Covered - 1
Properties of System
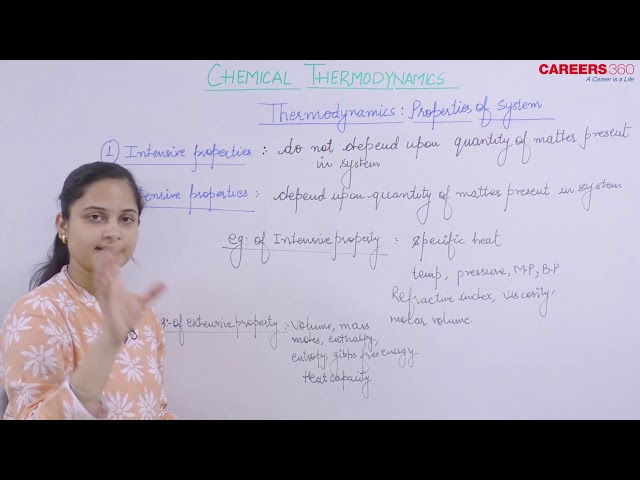
All macroscopic properties of a system irrespective of the fact whether they are state variable or not are divided into two types:
1. Intensive Properties
Such properties remain the same on any division in the system that is, do not depend upon the amount of substance present in the system.
Example: Temperature, pressure, concentration, density, viscosity, surface tension, specific heat, refractive index, pH, EMF of dry cell, vapour pressure, dipole-moment etc.
2. Extensive Properties
Such properties depend upon the amount of substance that is, their values are different in the divided system than in the entire system
Example, Mass, volume, energy, work, internal energy, entropy, enthalpy, heat capacity, length.
An extensive property can be made intensive specifying it in unit amount of matter.
Example, Density $\approx\left(\frac{\text { Mass }}{\text { Volume }}\right)$
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"