Mayer's Formula - Practice Questions & MCQ
Quick Facts
-
Mayer's formula is considered one the most difficult concept.
-
39 Questions around this concept.
Solve by difficulty
An ideal gas has molecules with 5 degrees of freedom. The ratio of specific heat at constant pressure ( ) and at constant volume (
) is :
According to the law of equipartition of energy, the molar specific heat of a diatomic gas at a constant volume where the molecule has one additional vibrational mode is
The correct relation between and temperature
is :
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
Concepts Covered - 1
-
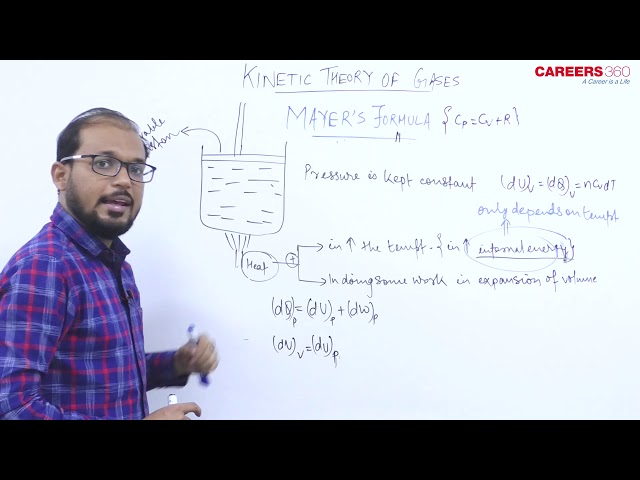
- Mayer's formula- As we know
Molar Specific heat of the gas at constant volume $=C_v$
and Molar Specific heat capacity at constant pressure $=C_p$
Mayer's formula gives the relation between $C_p$ and $C_v$ as $C_p=C_v+R$
or we can say that molar Mayer's formula shows that specific heat at constant pressure is greater than that at constant volume.
- Specific Heat in Terms of Degree of Freedom
1.Molar Specific heat of the gas at constant volume $\left(C_v\right)$For a gas at temperature $T$, the internal energy
$U=\frac{f}{2} n R T \Rightarrow$ Change in energy $\Delta U=\frac{f}{2} n R \Delta T \ldots$
Also, as we know for any gas heat supplied at constant volume$
(\Delta Q)_V=n C_V \Delta T=\Delta U \ldots \ldots(i i)
$
From the equation (i) and (ii)$
C_v=\frac{f R}{2}
$
where
f = degree of freedom
R= Universal gas constant
2. Molar Specific heat of the gas at constant pressure ( $C_p$ )
From Mayer's formula, we know that $C_p=C_v+R$
$
\Rightarrow C_P=C_V+R=\frac{f}{2} R+R=\left(\frac{f}{2}+1\right) R
$
3. Atomicity or adiabatic coefficient ( ${ }^{\prime}$ )
It is the ratio of $C_p$ to $C_v$
$
\gamma=\frac{C_p}{C_v}=1+\frac{2}{f}
$
Value of $\gamma$ is always more than 1
for Monoatomic gas
$
\gamma=\frac{5}{3}
$
for Diatomic gas
$
\gamma=\frac{7}{5}
$
for Triatomic gas
$
\gamma=\frac{4}{3}
$
- Gaseous Mixture
If two non-reactive gases A and B are enclosed in a vessel of volume V .
In the mixture $\mathrm{n}_1$ mole of Gas A (having Specific capacities as $C_{p 1}$ and $C_{v 1}$, Degree of freedom $f_1$ and Molar mass as $M_1$ ) is mixed with $\mathrm{n}_2$ mole of Gas B (having Specific capacities as $C_{p 2}$ and $C_{v 2}$, Degree of freedom $f_2$ and Molar mass as $M_2$ )
Then Specific heat of the mixture at constant volume will be
$
C_{v_{m i x}}=\frac{n_1 C_{v_1}+n_2 C_{v_2}}{n_1+n_2}
$
Similarly, Specific heat of the mixture at constant pressure will be
$
C_{p_{m i x}}=\frac{n_1 C_{p_1}+n_2 C_{p_2}}{n_1+n_2}
$
And adiabatic coefficient $(\gamma)$ of the mixture is given by
$
\gamma_{\text {mixure }}=\frac{C_{p_{m i x}}}{C_{v_{m x}}}=\frac{\frac{\left(n_1 C_{p_1}+n_2 C_{p_2}\right)}{n_1+n_2}}{\frac{\left(n_1 C_{v_1}+n_2 C_{v_2}\right)}{n_1+n_2}}=\frac{\left(n_1 C_{p_1}+n_2 C_{p_2}\right)}{\left(n_1 C_{v_1}+n_2 C_{v_2}\right)}
$
Also
$
\frac{1}{\gamma_{\operatorname{mix}}-1}=\frac{\frac{n_1}{\gamma_1-1}+\frac{n_2}{\gamma_2-1}}{n_1+n_2}
$
Similarly, the Degree of freedom of mixture is given as
$
f_{\operatorname{mix}}=\frac{n_1 f_1+n_2 f_2}{n_1+n_2}
$
Similarly, the molar mass of the mixture
$
M_{m i x}=\frac{n_1 M_1+n_2 M_2}{n_1+n_2}
$
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"