De-broglie's Explanation Of Bohr's Second Postulate - Practice Questions & MCQ
Quick Facts
-
De-broglie's explanation of Bohr's second postulate is considered one of the most asked concept.
-
12 Questions around this concept.
Solve by difficulty
The acceleration of an electron in the first orbit of the hydrogen atom (n=1) is:
Suppose an electron is attracted towards the origin by a force k / r where k is constant and r is the distance of the electron from the origin. By applying the Bohr model to this system, the radius of the nth orbital of the electron is found to be rn and the kinetic energy of the electron to be Tn. Then which of the following is true?
If the capacitance of a nano-capacitor is measured in terms of a unit made by combining the electronic charge
, Bohr radius'a0', Planck's constant 'h' and speed of light 'c' then :
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
A small particle of mass m moves in such a way that its potential energy is constant and r is the distance of the particle from the origin. Assuming Bohr's quantization of momentum and circular orbit, the radius of the nth orbit will be proportional to,
A particle of mass $m$ moves around the origin in a potential $\frac{1}{2} m w^2 r^2$, where r is the distance from the origin. Applying the Bohr model in this case, the radius of the particle in its nth orbit in terms of $a=\sqrt{h /(2 \pi m \omega)}$ is:
Concepts Covered - 1
De-broglie's explanation of Bohr's second postulate-
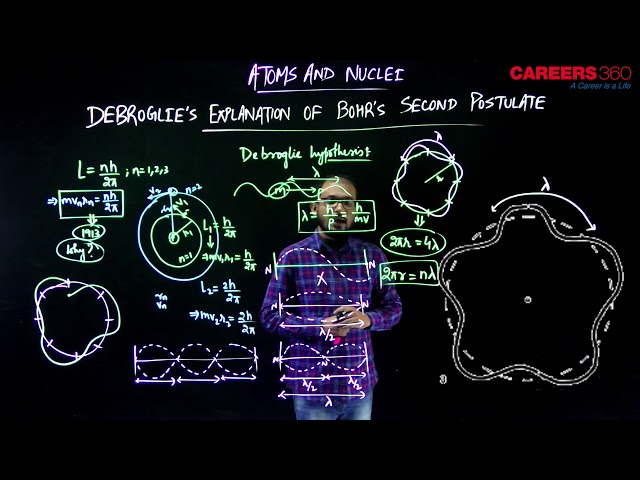
Since the Bohr gave many postulates in his theory, but the second postulate is not very clear and little puzzling. The Scientist De Broglie explained this puzzle very clearly that why the angular momentum of the revolving electron is the integral multiple of the $h / 2 \pi$. De broglie in his experiment proved that the electron revolving the circular orbit has wave nature also in the last chapter we have seen the experiment performed by the Davison and Germer which proved that the electron shows the wave nature. In analogy to waves travelling on a string, particle waves too can lead to standing waves under resonant conditions. During the chapter Waves and Oscillation, we know that when a string is plucked, a vast number of wavelengths are excited. However only those wavelengths survive which have nodes at the ends and form the standing wave in the string. It means that in a string, standing waves are formed when the total distance travelled by a wave down the string and back is any integral number of wavelengths. Waves with other wavelengths interfere with themselves upon reflection and their amplitudes quickly drop to zero.
For an electron moving in $n^{\text {th }}$ circular orbit of radius $r_n$, the total distance is the circumference of the orbit, $2 \pi r_n$.
$2 \pi r_n=n \lambda, \quad n=1,2,3 \ldots$

Figure given above illustrates a standing particle wave on a circular orbit for $\mathrm{n}=4$, i.e., $2 \pi r_n=4 \lambda$, where $\lambda$ is the de Broglie wavelength of the electron moving in $\mathrm{n}^{\text {th }}$ orbit. From the last chapter we have studied that $h=h / p$, where p is the magnitude of the electron's momentum. If the speed of the electron is much less than the speed of light, the momentum is $m v_n$.
Thus,
$
\lambda=\frac{h}{m v_n}
$
From the above equation, we have,
$
2 \pi r_n=\frac{n h}{m v_n} \quad \text { or }, \quad m v_n r_n=\frac{n h}{2 \pi}
$
This is the quantum condition proposed by Bohr for the angular momentum of the electron. Thus de Broglie hypothesis provided an explanation for Bohr’s second postulate for the quantisation of angular momentum of the orbiting electron.
The quantised electron orbits and energy states are due to the wave nature of the electron and only resonant standing waves can persist. Bohr’s model, involving classical trajectory picture (planet-like electron orbiting the nucleus), correctly predicts the gross features of the hydrogenic atoms(Hydrogenic atoms are the atoms consisting of a nucleus with positive charge +Ze and a single electron, where Z is the proton number. Examples are hydrogen atom, singly ionised helium, doubly ionised lithium, and so forth.), in particular, the frequencies of the radiation emitted or selectively absorbed.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"