Amity University-Noida B.Tech Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Mass-energy equivalence and Nuclear binding energy, Binding Energy Per Nucleon, Nuclear Force and Stability is considered one of the most asked concept.
66 Questions around this concept.
If is the mass of an oxygen isotope
are the masses of a proton and a neutron respectively, the nuclear binding energy of the isotope is
A nucleus disintegrates into two nuclear parts which have velocities in the ratio 2:1. The ratio of their nuclear sizes will be
The above is a plot of binding energy per nucleon $E_{b,}$ against the nuclear mass $M$; $A, B, C, D, E, F$ correspond to different nuclei. Consider four reactions:

(i) $A+B \rightarrow C+\varepsilon$
(ii) $C \rightarrow A+B+\varepsilon$
(iii) $D+E \rightarrow F+\varepsilon$
(iv) $F \rightarrow D+E+\varepsilon$
where $\varepsilon$ is the energy released? In which reactions is $\varepsilon$ positive?
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
Question is based on the following paragraph.
A nucleus of mass $M+\Delta m$ is at rest and decays into two daughter nuclei of equal mass $\frac{M}{2}$ each. Speed of light is $c$.
Question: The binding energy per nucleon for the parent nucleus is $E_1$ and that for the daughter nuclei is $E_2$.Then
If Rydberg's constant is R then the wave number in the second bracket series is -
Direction: In the following question, a statement of Assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion: Mass of a particle can be converted into energy.
Reason: For mass defect $\Delta m$ energy $\mathrm{E}=\Delta m c^2$
The energy equivalent of $1 \mathrm{~g}$ of substance is:
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
In the nuclear reaction given by ${ }_2 \mathrm{He}^4+{ }_7 \mathrm{~N}^{14} \rightarrow{ }_1 \mathrm{H}^1+\mathrm{X}$ then the nucleus X is :
When nuclei are bombarded by protons, and the resultant nuclei are
the emitted particles will be :
An alpha nucleus of energy bombards a heavy nuclear target of charge Ze. Then the distance of the closest approach for the alpha nucleus with be proportional to
Energy mass equivalence-
Einstein showed from his theory of special relativity that it is necessary to treat mass as another form of energy.
Einstein showed that mass is another form of energy and one can convert mass into other forms of energy, say kinetic energy and vice-versa.
For this Einstein gave the famous mass-energy equivalence relation
$E=m c^2$ where c is the velocity of light in vacuum and $c=3 \times 10^8 \mathrm{~m} / \mathrm{s}$ or we can say $\Delta E=\Delta m c^2$
where $\Delta m=$ mass defect and $\Delta E=$ energy released
Note:
| Rest mass of an electron (me) | 9.1 x 10-31 kg |
| 5.485 x 10-4 amu | |
| Rest mass of a proton (mp) | 1.6726 x 10-27 kg |
| 1.00727 amu | |
| 1836.15 me | |
| Rest mass of a neutron (mn) | 1.6749 x 10-27 kg |
| 1.0086 amu | |
| Energy equivalence of rest mass of an electron | 0.51 MeV |
| Energy equivalence of rest mass of a proton | 938.27 MeV |
| Energy equivalence of rest mass of a neutron | 939.56 MeV |
It is very useful to calculate energy emitted in the nuclear process.
Mass defect-
It is found that the mass of a nucleus is always less than the sum of masses of its constituent nucleons in a free state.
This difference in masses is called the mass defect.
Hence mass defect is given as
$\Delta m=$ Sum of masses of nucleons- Mass of the nucleus
$\Delta m=\left[Z m_p+(A-Z) m_n\right]-M$
where
$m_p=$ Mass of proton, $m_n=$ Mass of each neutron,
$M=$ Mass of nucleus, $Z=$ Atomic number, $A=$ Mass number
Note- The mass of a typical nucleus is about $1 \%$ less than the sum of masses of nucleons.
Packing fraction -
Mass defect per atomic mass number is called packing fraction.
Packing fraction measures the stability of a nucleus. The smaller the value of the packing fraction, the larger is the stability of the nucleus.
Packing fraction $(f)=\frac{\Delta m}{A}=\frac{M-\left(Z m_p+(A-Z) m_n\right)}{A}$
$m_p=$ Mass of proton, $m_n=$ Mass of each neutron,
$M=$ Mass of nucleus, $Z=$ Atomic number, $A=$ Mass number
Packing Fraction can have positive, negative, or zero value.
Zero value of packing fraction is found in monoisotopic elements where the isotopic mass is equal to the mass number. For $8 \mathrm{O}^{16}$, $f \rightarrow$ zero
Negative value of packing fraction indicates that there is a mass defect, hence binding energy. Such nuclei are stable.
Positive values of Packing fraction are unstable undergoing fission and fusion processes.
Nuclear binding energy (B.E)-
The neutrons and protons in a stable nucleus are held together by nuclear forces and energy is needed to pull them infinitely apart. This energy is called the binding energy of the nucleus.
OR
Amount of energy released when nucleons come together to form a nucleus is called the binding energy of the nucleus.
OR
The binding energy of a nucleus may be defined as the energy equivalent to the mass defect of the nucleus.
If $\Delta m$ is a mass defect then according to Einstein's mass-energy relation
then
To see how nucleon binding energy varies from nucleous to nucleous, it is important to compare the binding energy on nucleon basis. A more useful measure of the binding between protons and neutrons is the binding energy per nucleon or $\mathrm{E}_{b n}$. It is the ratio of the binding energy of a nucleus to the number of nucleons in the nucleus:
$$
\mathrm{E}_{\mathrm{bn}}=\frac{\mathrm{E}_{\mathrm{b}}}{\mathrm{~A}} \quad \text { or } \quad \mathrm{E}_{\mathrm{bn}}=\frac{\Delta \mathrm{Mc}^2}{\mathrm{~A}}
$$
where, A = Number of Nucleons.
We can define binding energy per nucleon theoretically as the average energy per nucleon needed to separate a nucleus into its individual nucleons.
Let’s look at a plot of the binding energy per nucleon versus the mass number for a large number of nuclei:

Following main features of the plot are:
Conclusion 1
The force is attractive and sufficiently strong to produce a binding energy of a few MeV per nucleon.
Conclusion 2
Conclusion 3
A very heavy nucleus, say $A=240$, has lower binding energy per nucleon compared to that of a nucleus with $A=120$. Thus if a nucleus $A=240$ breaks into two $A=120$ nuclei, nucleons get more tightly bound. Also, in the process energy is released. This concept is used in Nuclear Fission.
Conclusion 4
Now consider two very light nuclei with $A<10$. If these two nuclei were to join to form a heavier nucleus, then the binding energy per nucleon of the fused and heavier nucleus would be more than the $\mathrm{E}_{\mathrm{bn}}$ of the lighter nuclei. So, the nucleons are more tightly bound post-fusion. Again energy would be released in such a process of fusion. This is the energy source of the sun,
Nuclear Force
Coulomb force is a force that determines the motion of atomic electrons. As in the previous concept, we have seen that for average mass nuclei the binding energy per nucleon is approximately 8 MeV, This is much larger than the binding energy in atoms. Hence, the nuclear force required to bind a nucleus together must be very strong and of a different type. It must be strong enough to overcome the repulsion between the (positively charged) protons and to bind both protons and neutrons into the tiny nuclear volume.
Le's look at the features of this force also called the nuclear binding force which is obtained from many experiments which were performed in between 1930 and 1950.
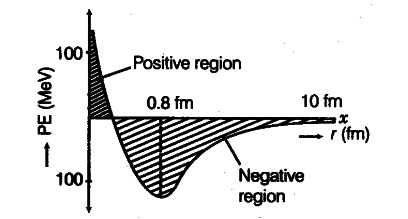
The potential energy of two nucleons is a function of the distance between them.
If distance $>r_\alpha$, then nuclear force $=$ attractive
If distance $<r_\alpha$ the nuclear force $=$ repulsive
Nuclear Stability
Nuclear Stability is a concept that helps to identify the stability of an isotope. The two main factors that determine nuclear stability are the neutron/proton ratio (neutron to proton ratio.) and the total number of nucleons in the nucleus.
NEUTRON/PROTON RATIO
The graph below is a plot of the number of neutrons versus the number of protons in various stable isotopes. Stable nuclei with atomic numbers up to about 20 have an n/p ratio of about 1/1.
Above Z = 20, the number of neutrons always exceeds the number of protons in stable isotopes. The stable nuclei are located in the pink band known as the belt of stability. The belt of stability ends at lead-208.
NUMBER OF NUCLEONS
No nucleus higher than lead-208 is stable. That's because, although the nuclear strong force is about 100 times as strong as the electrostatic repulsions, it operates over only very short distances. When a nucleus reaches a certain size, the strong force is no longer able to hold the nucleus together.
"Stay in the loop. Receive exam news, study resources, and expert advice!"
