X-rays - Practice Questions & MCQ
Quick Facts
-
8 Questions around this concept.
Solve by difficulty
In radio therapy, x-rays are used to -
Direction : In the following question, a statement of Assertion ( A ) is followed by a statement of reason (R). Mark the correct choice as
Assertion : X-ray can not obtain from hydrogen atom
Reason : Hydrogen atom has single electron
Concepts Covered - 1
X-rays-
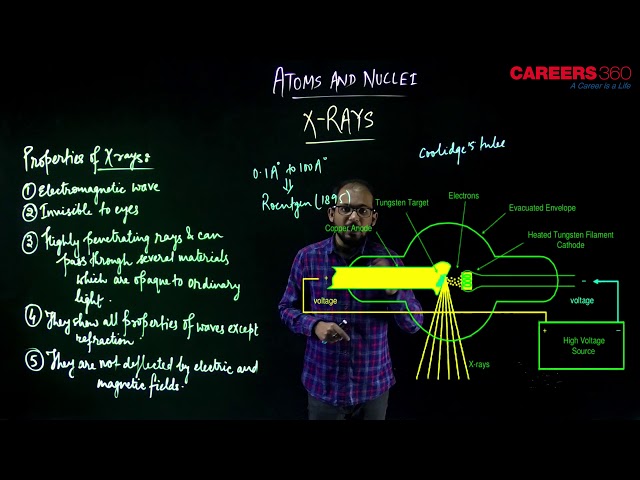
X-rays are highly energetic radiations with very short wavelengths. Their wavelength is even shorter than the ultraviolet radiations and varies between 0.03 and 3 nanometers and some x-rays are as small as a single atom of many elements. But now the question arises that how X-rays were discovered, Actually X-rays were discovered by Roentgen, who found that a discharge tube(Coolidge's tube), operating at low pressure and high voltage, emitted radiation that caused a fluorescent in the neighborhood to glow brightly. This indicates that some unknown radiation was responsible for fluorescence. Since the name of these rays was unknown so it is named X-rays.
Properties of X-rays -
-
They pass through materials more or less unchanged
-
They cannot be refracted
-
Electric and magnetic fields do not have any effect on these rays
-
These radiations ionize the surrounding air by discharging electrified bodies
-
They have a short wavelength varying between $0.1 \mathrm{~A}^{\circ}$ to $1 \mathrm{~A}^{\circ}$.
-
They are produced when a metal anode is bombarded by very high-energy electrons.
-
They do not require any medium for propagation
-
X-rays cannot be focused on a single point
-
These radiations cannot be heard or smelt
-
They travel in a straight line in free space
-
They cause photoelectric emissions.
-
The intensity of X – rays depends on the number of electrons hitting the target.
Application of X-rays -
- Medical Science - They are used for medical purposes to detect the breakage in human bones.
- Security - They are used as a scanner to scan the luggage of passengers in airports, rail terminals, and other places.
- Astronomy - It is emitted by celestial objects and is studied to understand the environment.
- Industry - It is widely used to detect defects in welds.
- Restoration - They are used to restore old paintings.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"