Resonance Energy - Practice Questions & MCQ
Quick Facts
-
4 Questions around this concept.
Solve by difficulty
The difference in energy between the actual structure and the lowest energy resonance structure for the given compound is :
Concepts Covered - 1
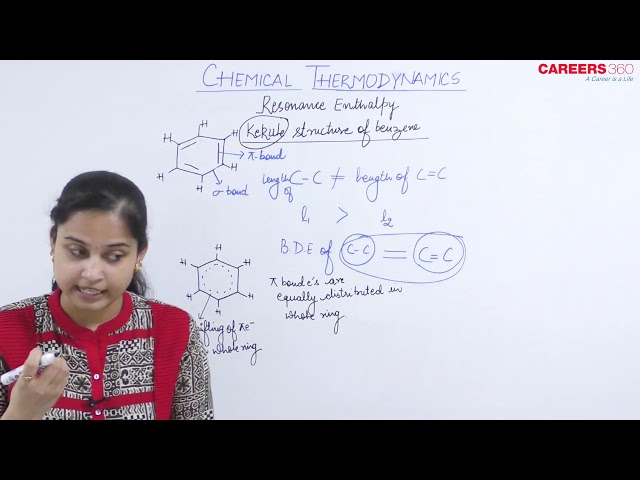
Resonance Enthalpy:
The theoretical difference in molecular energy between a resonance hybrid and the 'most stable' resonance contributor (if this resonance contributor existed as a real molecule). In other words, the stability gain by electron delocalization due to resonance versus the absence of such delocalization. The resonance energy of benzene is 36 kcal mol-1 .
Calculation of Resonance Energy:
(1) Resonance energy can be calculated in terms of the heat of the formation data:
Resonance Energy $=\Delta_{\mathrm{f}} \mathrm{H}_{\text {theoretical }}^{\circ}-\Delta_{\mathrm{f}} \mathrm{H}_{\text {actual }}^{\circ}$
(2) Resonance energy can be calculated in terms of the heat of combustion data:
Resonance Energy $=\Delta_c \mathrm{H}_{\text {actual }}^{\circ}-\Delta_c \mathrm{H}_{\text {theoretical }}^{\circ}$
(3) Resonance energy can be calculated in terms of the heat of hydrogenation data:
Resonance Energy $=\Delta_{\text {hydrogenation }} \mathrm{H}_{\text {actual }}^{\circ}-\Delta_{\text {hydrogenation }} \mathrm{H}_{\text {theoretical }}^{\circ}$
Note: Resonance Energy is always a Positive Number. Use this fact to quickly calculate Resonance Energy using the above formulae.
The calculation of Resonance energy in terms of Heat of hydrogenation is given below:

Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"