pH Of Acids And Bases - Practice Questions & MCQ
Quick Facts
-
pH of Solutions: Strong Acids, pH of Solutions: Weak Acids, pH of solution/mixture are considered the most difficult concepts.
-
40 Questions around this concept.
Solve by difficulty
Which one of the following statements is not true?
Among the following acids which has the lowest value?
When the hydrogen ion concentration changes by a factor of 1000 , the value of
of the solution
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
The pH of 0.1 M aqueous solutions of NaCl, CH3COONa and NH4Cl will follow the order
The $p H_{\text {values of }}(i) 0.1 \mathrm{MHCl}_{a q}(i i) 0.1 \mathrm{MKOH},(i i i)_{\text {tomato juice and }}(i v)_{\text {pure water }}$ follow the order
Concepts Covered - 4
pH is also referred to as potential or power of hydrogen. Mathematically, it can be represented as follows:
If solution is neutral, then:
Kw = [H3O+][OH-]
From the ionic product of water, we know:
Kw = 10-14
[H3O+] = [OH-] = x (since solution is neutral)
Thus, 10-14 = Kw = x2
x = 10-7
Now, [H3O+] = 10-7
Thus, pH = - log10(H3O+) = - log10(10-7) = 7
For Acidic solutions: For Basic solutions:
For acidic solutions, we must have [H3O+] > [OH-] For basic solutions, we must have [H3O+] < [OH-]
Thus, [H3O+] > 10-7 Thus, [H3O+] < 10-7
Thus, [H3O+] for acids can be 10-6, 10-5, 10-4, etc. Thus, [H3O+] for basics can be 10-8, 10-9, 10-10, etc.
Thus, pH of acids can be 6, 5, 4, etc. Thus, pH of basics can be 8, 9, 10, 11, etc.
Hence, pH of acidic solutions is less than 7 Hence, pH of basic solutions is greater than 7
pH depends upon temperature
We know from ionic product of water that at 630C, the value of Kw = 10-13.
For neutral solution we know:
Hence, pH depends upon temperature
pH of Strong Acids
Strong acids are those acids which dissociate completely in solutions. For example:
- 2 x 10-3 M HNO3
Since HNO3 is a strong acid, thus it will dissociate completely into H+ and OH- ions as follows:
Thus, pH of HNO3 is 2.7
- 10-4 M H2SO4
Since H2SO4 is a strong acid, thus it will dissociate completely into H+ and OH- ions as follows:
Thus, pH of H2SO4 is 3.7
NOTE: If molarity(N) of solution is not given but normality(N) is given, then molarity can be calculated using the following formula:
N = M x n
where, n is the number of moles
pH of Weak Acids
Weak acids are those acids which dissociate partially in solutions. For example:
- 8 M HA (Ka =2 x 10-8)
The chemical equation for the dissociation of weak acid HA is as follows:
Initial: 8M 0 0
Equil: 8 - 8𝛂 8𝛂 8𝛂
The equilibrium constant Ka for the weak acid is given as follows:
Thus, pH of this given acid = 3.4
- 0.002N CH3COOH(𝛂 = 0.02)
The chemical equation for the dissociation of CH3COOH is as follows:
Initial: c 0 0
Equil: c - c𝛂 c𝛂 c𝛂
The equilibrium constant Ka for the weak acid is given as follows:
Thus, pH of acetic acid = 4.4
Strong bases
Strong bases are those bases that dissociate completely in solution. For example:
- 0.1M NaOH
Since NaOH is a strong base, thus it will dissociate completely into Na+ and OH- ions. The chemical equation for the dissociation of NaOH is as follows:
Hence, pH of 0.1M NaOH solution is 13.
- 0.05M Ba(OH)2
Since Ba(OH)2 is a strong base, thus it will dissociate completely into Ba2+ and 2OH- ions. The chemical equation for the dissociation of Ba(OH)2 is as follows:
Hence, pH of 0.05M Ba(OH)2 solution is 13.
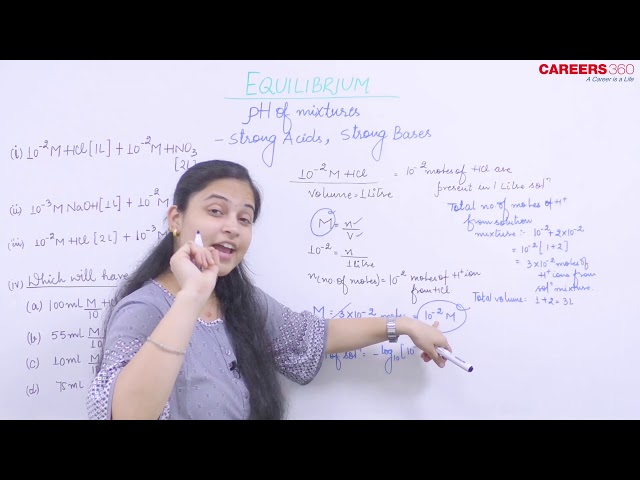
- Mixture of Strong Acids:
NOTE: Shortcut only for monobasic acids and monoacidic bases:
- Mixture of Strong Bases:
Using the shortcut formula for bases as given above, we get:
- Mixture of Strong Acid and Strong Base:
Clearly, moles of (H+) = 2 x 10-2 moles and moles of (OH-) = 1 x 10-3 moles.
Since moles of (H+) is greater than moles of (OH-), therefore the solution medium will be acidic.
Now, remaining moles of H+ = 2 x 10-2 - 10-3 = 19 x 10-3
Thus, pH of the mixture = 2.2
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"