Second Order Reaction - Practice Questions & MCQ
Edited By admin | Updated on Sep 18, 2023 18:35 AM | #JEE Main
Quick Facts
-
19 Questions around this concept.
Solve by difficulty
In a second-order reaction. How does the rate change when the concentration of a reactant is doubled?
Which among the following graphs pertains to a Second order reaction?
Concepts Covered - 1
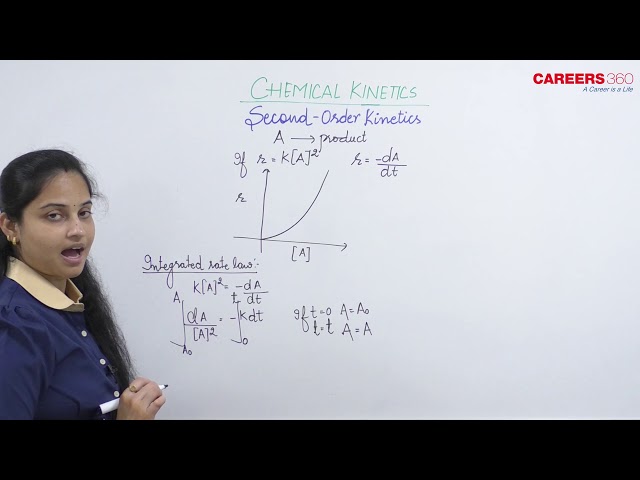
Second Order Kinetics
Consider the reaction
$\mathrm{A} \rightarrow \mathrm{P}$
For second-order reaction, the rate law is given as follows:
Rate $=\mathrm{k}[\mathrm{A}]^2=-\frac{\mathrm{dA}}{\mathrm{dt}}$
- Integrated Rate Law: Since it is a second-order reaction, thus:
$\frac{-\mathrm{dA}}{\mathrm{dt}}=\mathrm{k}[\mathrm{A}]^2$
Integrating both sides, we get:
$\int_{\mathrm{A}_{\mathrm{o}}}^{\mathrm{A}} \frac{\mathrm{dA}}{\mathrm{A}^2}=-\int_0^{\mathrm{t}} \mathrm{kdt}$
where Ao is the initial concentration of A at time t = 0
A is the remaining concentration of A after time 't'
$\Rightarrow\left[\frac{-1}{\mathrm{~A}}\right]_{\mathrm{A}_{\mathrm{o}}}^{\mathrm{A}}=-\mathrm{k}(\mathrm{t})_0^{\mathrm{t}}$
$\Rightarrow\left[\frac{1}{\mathrm{~A}}\right]_{\mathrm{A}_{\mathrm{o}}}^{\mathrm{A}}=\mathrm{kt}$
$
\Rightarrow \frac{1}{\mathrm{~A}}-\frac{1}{\mathrm{~A}_{\mathrm{o}}}=\mathrm{kt}
$
Thus, $\frac{1}{[\mathbf{A}]}=\mathbf{k t}+\frac{\mathbf{1}}{\left[\mathbf{A}_{\mathbf{o}}\right]}$ - Half-life(t1/2): We know that the half-life for a reaction is the time when the concentration of the reactant(A) is half of its initial value.
Thus, at time t = t1/2, A = Ao/2
From equation (i) we have:
$\mathrm{t}=\frac{1}{\mathrm{k}}\left[\frac{1}{\mathrm{~A}}-\frac{1}{\mathrm{~A}_{\mathrm{o}}}\right]$
$\Rightarrow \mathrm{t}_{1 / 2}=\frac{1}{\mathrm{k}}\left[\frac{1}{\mathrm{~A}_{\mathrm{o}} / 2}-\frac{1}{\mathrm{~A}_{\mathrm{o}}}\right]$
Thus, $\mathbf{t}_{1 / 2}=\frac{1}{\mathrm{kA}_{\mathrm{o}}}$
This is the half-life for the second-order reaction.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"