Gibbs Free Energy of Reaction - Practice Questions & MCQ
Quick Facts
-
32 Questions around this concept.
Solve by difficulty
The values of for the reaction,
are 170 kJ and 170
, respectively. The reaction will be spontaneous at
Given
,
,
Among the following, the strongest reducing agent is :
Statement I:- There will be no effect of catalyst on Gibbs energy
Statement II:- Activation energy increase by Catalyst
Choose Correct option.
JEE Main 2026: January Question Paper with Solutions
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Important Formulas | Foreign Universities in India
Concepts Covered - 1
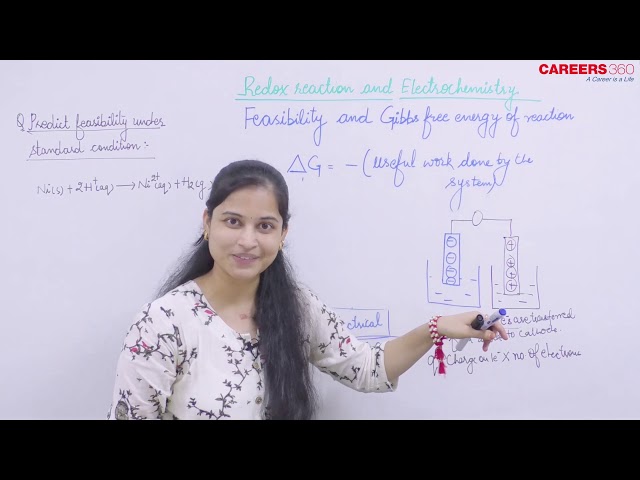
Let n faraday charge be involved in a cell generating an emf E, then the magnitude of the work done by the cell will be calculated as:
|Work| = Charge × Potential = $(n F) \times(E)$
Now, the maximum work that can be extracted from the cell is equal to the decrease in the Gibb's free energy.
So, it can be said that
$-\Delta \mathrm{G}=\mathrm{nFE}$
The negative sign is incorporated to include the spontaineity relation between E and Gibb's free Energy
Similarly, maximum obtainable work from the cell at standard condition will be:
$\mathrm{W}_{\max }=\mathrm{nFE}_{\text {cell }}^0 \quad$ where $\mathrm{E}_{\text {cell }}^0=$ standard emf of standard cell potential
$-\Delta \mathrm{G}^{\circ}=\mathrm{nFE} \mathrm{E}_{\text {cell }}^0$
Thus, for a spontaneous cell reaction,
E > 0 and $\Delta$G < 0
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"