Electronic Configuration in Periods and Groups - Practice Questions & MCQ
Quick Facts
-
14 Questions around this concept.
Solve by difficulty
Match List I with List II
| LIST I(Atomic Number) |
LIST II (Block of periodic table ) |
||
| A. | 37 | I. | p-block |
| B. | 78 | II. | d-block |
| C. | 52 | III. | f-block |
| D. | 65 | IV | s-block |
Choose the correct answer from the options given below:
Which of the following sets contain only isoelectronic ions?
(i) $\mathrm{Zn}^{2+}, \mathrm{Ca}^{2+}, \mathrm{Ga}^{3+}, \mathrm{Al}^{3+}$
(ii) $\mathrm{K}^{+}, \mathrm{Ca}^{2+}, \mathrm{Sc}^{3+}, \mathrm{Cl}^{-}$
(iii) $\mathrm{P}^{-3}, \mathrm{~S}^{2-}, \mathrm{Cl}^{-}, \mathrm{K}^{+}$
(iv) $\mathrm{Ti}^{4+}, \mathrm{Ar}, \mathrm{Cr}^{3+}, V^{5+}$
The element with atomic number 16 belongs to which group of the periodic table:
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
The element with the electronic configuration $[X e] 4 f^{14} 5 d^1 6 s^2$ belongs to which of the following group:
An element has the configuration $1 s^2, 2 s^2, 2 p^6, 3 s^2, 3 p^2$ : To which block in the long form of the periodic table, does this belong?
Concepts Covered - 1
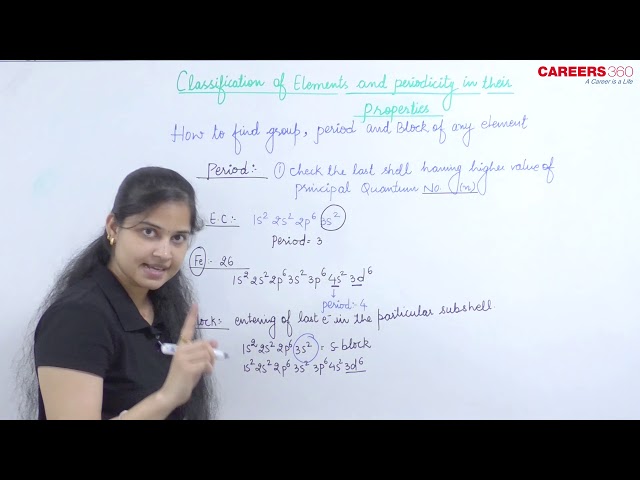
Period
The period of any element is determined by the last shell in which the last electrons enters. For example, Fe atomic number is 26. The electronic configuration can be written as:
1s22s22p63s23p64s23d6
Now, the last electron enters into the d-subshell but electrons are also present in 4th subshell. Therefore, Fe belongs to fourth period.
Block
The block of any element is determined by the last subshell in which the last electron enters. For example, Na has atomic number 11, thus its electronic configuration can be written as:
1s22s22p63s1
Now, its last electron enters into the s-subshell, therefore, Na belongs to the s-block.
Group
The group of any element is determined in different ways.
-
For s-block
If the last electron of any element enters into the s-subshell, then the group number is equal to the number of electrons in the last s-subshell.
For example, K has atomic number 19, thus its electronic configuration can be written as:
1s22s22p63s23p64s1
Now it has 1 electron in the s-subshell, therefore K belongs to Group 1.
-
For p-block
If the last electron of any element enters into the p-subshell, then the group number is equal to (12 + the number of electrons in the last p-subshell).
For example, Ge has atomic number 32, thus its electronic configuration can be written as:
1s22s22p63s23p64s23d104p2
Now, it has 2 electrons in the last p-subshell, therefore its group number is:
12 + 2 = 14
Thus, Ge belongs to Group 14
- For d-block
If the last electron of any element enters into the d-subshell, then the group number is equal to (2 + the number of electrons in (n-1)d-subshell.
For example, Mn has atomic number 25, thus its electronic configuration can be written as:
1s22s22p63s23p64s23d5
Now it has 5 electrons in the d-subshell, therefore its group number is:
2 + 5 = 7
Thus, Mn belongs to Group 7.
- For f-block
There are only two series of f-block i.e, lanthanide and actinide. If the last electron of any element enters into the f-subshell and if the atomic number is between 57-71, then the element belongs to lanthanide series i.e, 6th period. Further, if the last electron of any element enters into the f-subshell and if the atomic number is between 89-103, then the element belongs to the actinide series i.e, 7th period. All the elements from both these series belong to group 3.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"