Amity University-Noida B.Tech Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Preparation of Dihydrogen is considered one the most difficult concept.
Uses of Hydrogen is considered one of the most asked concept.
50 Questions around this concept.
$\mathrm{Zn}+2 \mathrm{NaOH} \rightarrow A+B$
A could be :
Reaction between following pairs will produce hydrogen except :
$\mathrm{CH}_{4(g))}+\mathrm{H}_2 \mathrm{O}_{(\mathrm{g})} \xrightarrow{1270 \mathrm{~K}} \mathrm{~A} ;$
what is A?
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
High purity Hydrogen is obtained by barium hydroxide solution between which metal electrodes?
Acetic acid reacts with sodium metal at room temperature to produce
Some statements about heavy water are given below:
(a) Heavy water is used as a moderator in nuclear reactors.
(b) Heavy water is more associated than ordinary water.
(c) Heavy water is more effective solvent than ordinary water.
Which of the above statements are correct?
At a particular temperature and atmospheric pressure, the solid and liquid phases of a pure substance can exist in equilibrium. Which of the following term defines this temperature?
(i) Normal melting point
(ii) Equilibrium temperature
(iii) Boiling point
(iv) Freezing point
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 28th Feb | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
H2O2 in pure state what does?
Alloys used for storage of H2 is/are
In the structure of diborane
Preparation of Hydrogen: There are a number of methods for preparing dihydrogen from metals and metal hydrides.

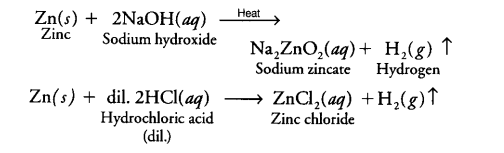
By the reaction of Zn with aqueous alkali : Hydrogen can also be prepared by the reaction of zinc with aqueous alkali.
Water Gas : Reaction of steam on hydrocarbons or coke at high temperatures in the presence of catalyst yields hydrogen.
The mixture of CO and H2 is called water gas. As this mixture of CO and H2 is used for the synthesis of methanol and a number of hydrocarbons, it is also called synthesis gas or 'syngas'.
The process of producing 'syngas' from coal is called 'coal gasification.
Water Gas Shift Reaction : The production of dihydrogen can be increased by reacting carbon monoxide of syngas mixtures with steam in the presence of iron chromate as catalyst.
This is called water-gas shift reaction.
Physical Properties of Hydrogen: Dihydrogen is a colourless, odourless, tasteless, combustible gas. It is lighter than air and insoluble in water. The electronegativity of hydrogen is in between metals and non-metals so it behaves as both electropositive and electronegative.
Disadvantage: A leak not only means a loss of hydrogen but is, in addition, a decided hazard because of the inflammability and very wide explosive limits that hydrogen possesses. These limits are much wider than for most gases.
The list of physical properties of Hydrogen and its isotopes are mentioned in the table below:

H-H bond Enthalpy : The H–H bond dissociation enthalpy is the highest for a single bond between two atoms of any element. It is because of this factor that the dissociation of dihydrogen into its atoms is only ~0.081% around 2000K which increases to 95.5% at 5000K. Also, it is relatively inert at room temperature due to the high H–H bond enthalpy.
Chemical Properties of Dihydrogen: Dihydrogen accomplishes reactions by
(i) loss of the only electron to give H+
(ii) gain of an electron to form H–
(iii) sharing electrons to form a single covalent bond.
The chemistry of dihydrogen can be illustrated by the following reactions:
This is the method for the manufacture of ammonia by the Haber process.
Reactions with metals: With many metals it combines at a high temperature to yield the corresponding hydrides
Hydrogen reacts with many organic compounds in the presence of catalysts to give useful hydrogenated products of commercial importance. For example :
The various uses of dihydrogen are as follows:
Ortho and Para hydrogens:
In a molecule of hydrogen when the spin of both H-atoms is in the same direction, they are known as ortho hydrogen.
In a molecule of hydrogen when the spin of both H-atoms is in the opposite direction, they are known as para hydrogen.

"Stay in the loop. Receive exam news, study resources, and expert advice!"
