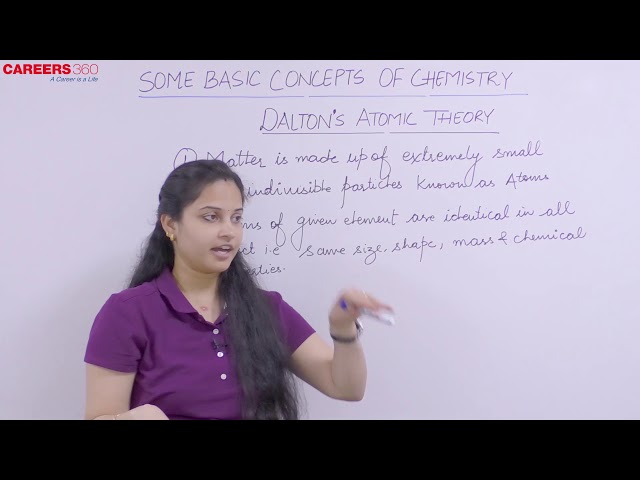- Engineering and Architecture
- Management and Business Administration
- Medicine and Allied Sciences
- Law
- Animation and Design
- Media, Mass Communication and Journalism
- Finance & Accounts
- Computer Application and IT
- Pharmacy
- Hospitality and Tourism
- Competition
- School
- Study Abroad
- Arts, Commerce & Sciences
- Learn
- Online Courses and Certifications
- Home
- JEE Main
- Study Material
- DALTON'S ATOMIC THEORY - Practice Questions & MCQ
DALTON'S ATOMIC THEORY - Practice Questions & MCQ
Quick Facts
-
11 Questions around this concept.
Solve by difficulty
Choose the Incorrect Statement about Dalton's Atomic Theory
Concepts Covered - 1
-
Matter consists of indivisible atoms.
-
Atom is indivisible and cannot be broken down.
-
All the atoms of a given element have identical properties, including identical mass. Atoms of different elements differ in mass.
-
Compounds are formed when atoms of different elements combine in a fixed ratio.
-
Chemical reactions involve the reorganization of atoms. These are neither created nor destroyed in a chemical reaction.
Study other Related Concepts
DALTON'S ATOMIC THEORY Current Topic
"Stay in the loop. Receive exam news, study resources, and expert advice!"

Books
Reference Books
DALTON'S ATOMIC THEORY
Chemistry Part I Textbook for Class XI
Page No. : 16
Line : 14
Clear your Basics with NCERT
E-books & Sample Papers

JEE Main 2026 January Session official Question Paper
5015+ Downloads

JEE Main 2026 April Attempt Strategy
4392+ Downloads
Get Answer to all your questions
B.Tech/B.Arch Admissions OPEN
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Amrita University B.Tech 2026
ApplyRecognized as Institute of Eminence by Govt. of India | NAAC ‘A++’ Grade | Upto 75% Scholarships
Vignan's Deemed to be University B.Tech Admissions 2026
Apply70th University Ranked by NIRF | 80th Engineering Rank by NIRF | Accredited by NBA and NAAC A+
UPES B.Tech Admissions 2026
ApplyLast Date to Apply: 26th March | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
MIT World Peace University B.Tech Admissions 2026
ApplyHighest CTC 44.14 LPA | UGC Approved | 1600+ Recruiters | 100% Placement
Manav Rachna-B.Tech Admissions 2026
ApplyNAAC A++ Grade | Recognized as Category-1 Deemed to be University by UGC | 41,000 + Alumni Imprints Globally
Explore on Careers360
Colleges By Branches
Colleges By Exam
BE/B.Tech
Diploma
Student Community: Where Questions Find Answers