Azeotropic Mixture - Practice Questions & MCQ
Quick Facts
-
30 Questions around this concept.
Solve by difficulty
Among the following mixtures, dipole-dipole as the major interaction, is present in
Example of maximum boiling azeotrope is:
Which of the following binary mixtures will have the same composition in the liquid and vapour phase?
(i) Benzene – Toluene
(ii) Water-Nitric acid
(iii) Water-Ethanol
(iv) n-Hexane – n-Heptane
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
If two liquids A and B form minimum boiling azeotrope at some specific composition then _______________.
On the basis of information given below mark the correct option.
Information:
(A) In bromoethane and chloroethane mixture intermolecular interactions of A–A and B–B type are nearly the same as A–B type interactions.
(B) In ethanol and acetone mixture A–A or B–B type intermolecular interactions are stronger than A–B type interactions.
(C) In chloroform and acetone mixture A–A or B–B type intermolecular interactions are weaker than A–B type interactions.
On the basis of information given below mark the correct option.
Information: On adding acetone to methanol some of the hydrogen bonds between methanol molecules break.
Concepts Covered - 2
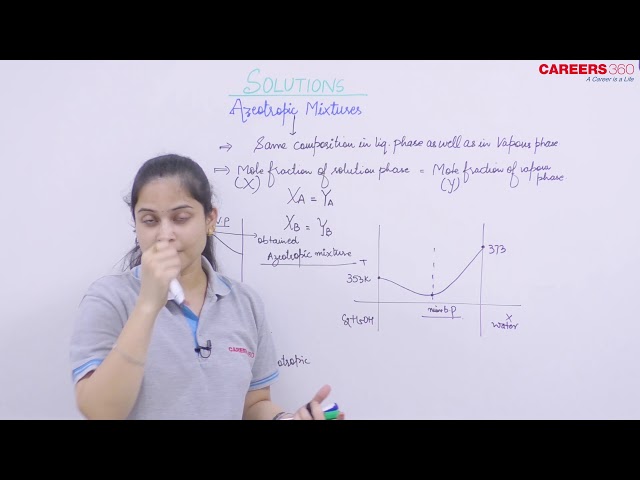
An azeotropic mixture is a solution of two liquids having a certain composition in which both the gas phase and the liquid phase composition are the same i.e. . These solutions distill over without changes in composition and hence, these cannot be separated by distillation. These solutions are formed by non-ideal solutions which show a large deviation from ideality.
These solutions boil at one particular temperature like a pure liquid and distills over in the same composition and hence are also referred to as constant boiling mixtures.
Types of Azeotropic Mixtures
It is of the following types:
-
Minimum Boiling Azeotropes
Non-ideal solutions showing large positive deviation from Raoult's law form minimum boiling azeotropes which boil at temperature lower than boiling point of either of the components 'A' or 'B'.
In the figure given below, the point M represents the azeotropic composition. At this point, vapour pressure is maximum and therefore the solution has lowest boiling point.

e.g., Ethanol water mixture on fractional distillation gives a solution containing approximately 95 % by volume of ethanol. Once this composition is achieved, no further separation occurs.
-
Maximum Boiling Azeotropes
Non-ideal solutions showing large negative deviation from Raoult's law form maximum boiling azeotropes which boil at temperature higher than the boiling point of either of the components 'A' or 'B'.
In the figure given below, the point B represents the azeotropic composition. At this point, vapour pressure is minimum and therefore the solution has the lowest boiling point.

e.g Nitric acid and water is an example of this class of azeotrope. This azeotrope has the approximate composition, 68% nitric acid and 32% water by mass
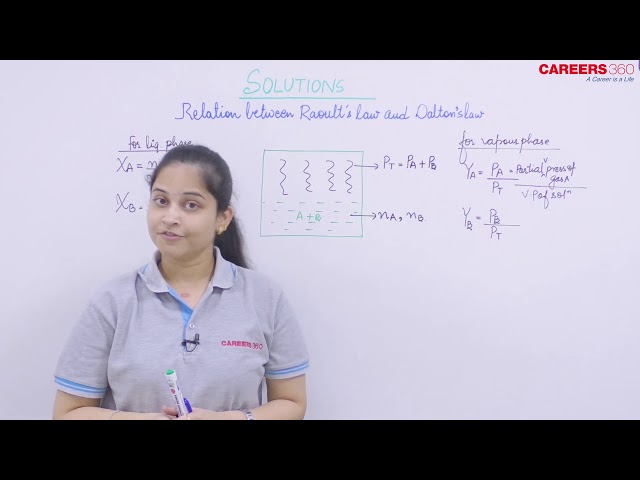
We have two liquids A and B and their vapour pressures are represented as PA and PB.
According to Raoult's law, we know:
Now, according to Dalton's law of partial pressure, we have:
Thus, on combining equations (i) with (iii) and (ii) with (iv), we get:
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"