Planck's quantum theory - Practice Questions & MCQ
Quick Facts
-
19 Questions around this concept.
Solve by difficulty
A gas absorbs a photon of 355 nm and emits at two wavelengths. If one of the emission is at 680 nm, the other is at
The energy required to break one mole of bonds in
. The longest wave-length of light capable of breaking a single
bond is
What is the wavelength of a photon with energy , according to Planck's quantum theory?
JEE Main 2026: January Question Paper with Solutions
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Important Formulas | Foreign Universities in India
The number of photons of light with the wavelength of 4000 pm that provides 1 J of energy is
The number of quanta of radiations of $V=4.67 \times 10^{13} \mathrm{~Hz}$ that must be absorbed in order to melt 5g of ice is (the energy required to melt 1g of ice is 333 J)
Concepts Covered - 1
Atom and molecules could emit (or absorb) energy only in discrete quantities known as quanta and not in a continuous manner. The following are certain phenomenon which could not be explained by the wave nature of electromagnetic radiation and are explained by the particle nature:
-
The nature of emission of radiation from hot bodies (black -body radiation).
-
Ejection of electrons from a metal surface when radiation strikes it (photoelectric effect).
-
Variation of heat capacity of solids as a function of temperature.
-
Line spectra of atoms with special reference to hydrogen.
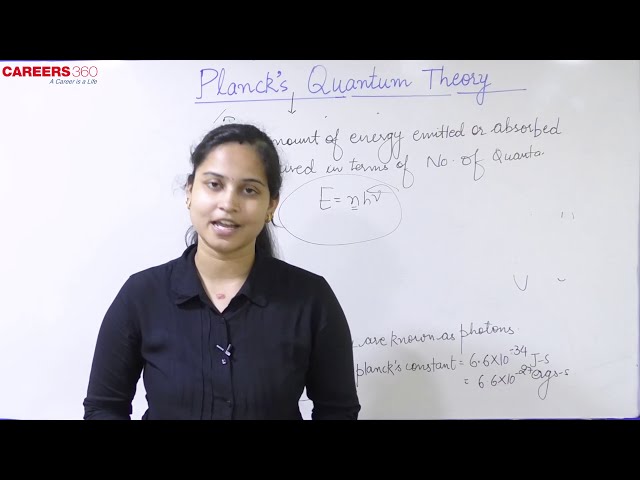
Max Planck suggested that the energy which is emitted or absorbed by the black body is not continuous but discontinuous in the form of small discrete packets of energy. Each such packet of energy is called a ‘quantum’. In case of light, the quantum of energy is called a ‘photon’. The energy of radiation is proportional to its frequency ($\nu$) and is expressed by the following equation:
$E=h \nu=\frac{h c}{\lambda}$
where h is the Planck's constant and it has a value equal to 6.63 $\times$ 10-34 J-s
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"