Amity University-Noida B.Tech Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Davisson-Germer Experiment is considered one of the most asked concept.
30 Questions around this concept.
Match List - I (Fundamental Experiment) with List - II (its conclusion) and select the correct option from the choices given below the list :

Match List - I (Experiment performed) with List - II (Phenomena discovered/ associated) and select the correct option from the options given below the lists :
| LIST I |
LIST II |
|
(a) |
Davisson and Germer Experiment |
(i) |
Wave nature of electrons |
|
(b) |
Millikan’s oil drop experiment | (ii) |
Charge of an electron |
|
(c) |
Rutherford experiment | (iii) |
Quantisation of energy levels |
|
(d) |
Franck -Hertz experiment | (iv) |
Existence of nucleus |
This question has statement 1 and statement 2 of the four choices given after the statements, choose the one that best describes the two statements
statement 1: Davisson – Germer experiment established the wave nature of electrons.
statement 2: It electrons have a wave nature, they can interfere and show diffraction.
JEE Main 2026: Result OUT; Check Now | Final Answer Key Link
JEE Main 2026 Tools: College Predictor
JEE Main 2026: Session 2 Registration Link | Foreign Universities in India
What is the wavelength of the incident beam of electrons in the Davisson-Germer experiment if the accelerating potential is 54 V?
In a Davisson Germer experiment, the distance between two adjacent crystal planes is . What is the angle of diffraction for constructive interference of electrons with a wavelength of
?
The wavelength of the X-rays is 0.071 nm which is diffracted by a plane of salt with 0.28 nm as the lattice constant. Determine the glancing angle for the second-order diffraction. Assume the value of the salt plane to be 110, and the given salt is rock salt.
Choose the correct statement regarding the Davisson Germer experiment:
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 26th March | Ranked #43 among Engineering colleges in India by NIRF | Highest Package 1.3 CR , 100% Placements
The lattice spacing in Ni crystal is equal to:
In a Davisson German experiment, the intensity of scattered beams of electron is found to be maximum when:
Davisson and Germer Experiment, for the first time, proved the wave nature of electrons through electron diffraction and also verified the de Broglie equation.
In this experiment, we will study the scattering of electrons by a Ni crystal.

The experimental setup for the Davisson and Germer experiment is enclosed within a vacuum chamber.
The experimental arrangement of the Davisson Germer experiment consists of the following main parts
Observations of Davisson Germer experiment-
These electrons formed a diffraction pattern. Thus the dual nature of matter was verified.
Note-Intensity of a scattered beam of electrons is found to be maximum when the angle of scattering is and the accelerating potential is 54 V.
i.e we could see a strong peak in the intensity. This peak was the result of the constructive interference of the scattered electrons.
The intensity of the scattered electrons is not continuous. It shows a maximum and a minimum value corresponding to the maxima and the minima of a diffraction pattern produced by X-rays.
Galvanometer in Davisson Germer Experiment-
The detector is connected to a sensitive galvanometer to measure the small values of current due to scattered beam of electrons.

The path difference between electrons scattered from adjacent crystal planes is given by $\Delta x=2 d \sin \theta$ and For constructive interference between the two scattered beams
$
\Delta x=2 d \sin \Theta=n \lambda
$
where $d$ - distance between diffracting planes
and $\theta$ is the angle between the incident rays and the surface of the crystal
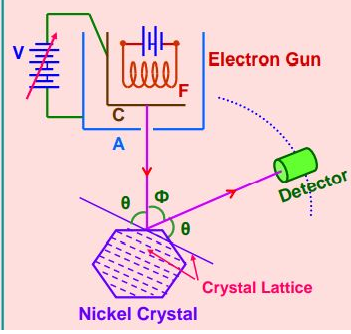
For the above figure $\phi=$ scattering angle
As $\theta+\phi+\theta=180^{\circ}$
${ }_{\text {So }} \Theta=\frac{180-\phi}{2}$
As Intensity of a scattered beam of electrons is found to be maximum when the angle of scattering is $50^{\circ}$
So For $\phi=50^{\circ}$ we get $\theta+50^{\circ}+\theta=180^{\circ}$
we get $\theta=65^{\circ}$
Co-relating Davisson Germer experiment and de Broglie relation-
According to de Broglie,
$
\lambda_e=\frac{12.27}{\sqrt{V}} A^{\circ}
$
and using $\mathrm{V}=54$ Volt we get $\lambda_e=0.167 \mathrm{~nm}$
From Bragg's formula we have $2 d \sin \Theta=n \lambda$
The Lattice Spacing in Ni Crystal is given as $\mathrm{d}=0.092 \mathrm{~nm}$.
And using $n=1, \phi=50^{\circ}$ and $V=50$ Volt
we get $\lambda_e=0.165 \mathrm{~nm}$
Therefore the experimental results are in a close agreement with the theoretical values got from the de Broglie equation.
Thus Davisson and Germer Experiment verify the de Broglie equation.
Frank Hertz Experiment-
This experiment is the first experimental verification of the existence of discrete energy states in atoms.
For this experiment, the graph of Collected current Vs Accelerating Voltage is given below
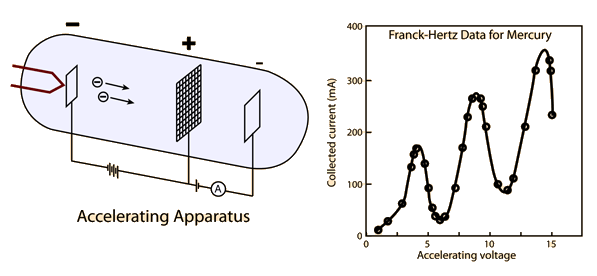
Frank and Hertz had proposed that the 4.9 V characteristic of their experiments was due to the ionization of mercury atoms by collisions with the flying electrons emitted at the cathode.
"Stay in the loop. Receive exam news, study resources, and expert advice!"
