Van Der Waals Forces - Practice Questions & MCQ
Quick Facts
-
8 Questions around this concept.
Solve by difficulty
In physisorption, adsorbent does not show specificity for any particular gas because ______________.
Concepts Covered - 1
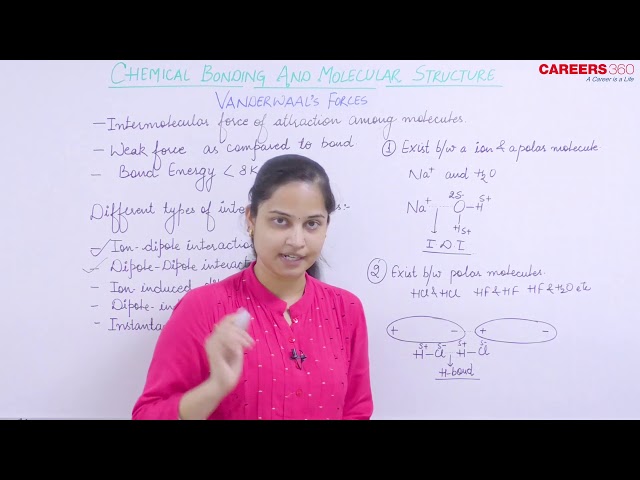
Van der Waal Forces
Van der Waal force of attraction is the force of attraction between the molecules. This force is weaker compared to bonds like covalent and ionic bonds.
Van der Waal forces can be divided into various categories as follows:
-
Ion-dipole interaction: This type of interaction exists between an ion and a polar molecule like HF, HCl, H2O, etc. The ion can be like Na+. This type of interaction is responsible for the dissolution of ions in solution.
-
Dipole-Dipole interaction: This type of interaction exists between two or more polar molecules. These dipoles can be H-Cl and H-Cl, NH3 and NF3, etc. Hydrogen bonding is a special type of dipole-dipole interaction.
-
Ion-Induced dipole interaction: This type of interaction exists between the ion and non-polar molecule. The charge on the ion causes the distortion in the electron cloud of the non-polar molecule and thus induces a dipole in the non-polar molecule. Then the ion and the induced-dipole attract each other.
-
Dipole-Induced dipole interaction: This kind of interaction exists between a polar and a non-polar molecule. For example, CCl4 in H2O. One of the dipole molecules distorts the electron cloud in the non-polar molecule and thus creates the dipole in non-polar as well.
-
Instantaneous dipole-dipole interaction: This type of interaction exists between two non-polar molecules. This force is also known as London forces. At any instant, electrons in one non-polar molecule come closer to each other and then this molecule becomes a dipole for instance. This instantaneous dipole distorts the electron cloud in another non-polar molecule and thus both behave like polar molecules. For example CCl4 and CCl4.
The strength of these forces follows the given order:
Ion-Dipole > Dipole-Dipole > Ion-Induced Dipole > Dipole-Induced Dipole > London Forces
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"